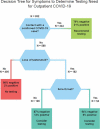
Proposed clinical indicators for efficient screening and testing for COVID-19 infection using Classification and Regression Trees (CART) analysis
- PMID: 33079625
- PMCID: PMC8023244
- DOI: 10.1080/21645515.2020.1822135
Proposed clinical indicators for efficient screening and testing for COVID-19 infection using Classification and Regression Trees (CART) analysis
Abstract
The introduction and rapid transmission of SARS-CoV-2 in the United States resulted in methods to assess, mitigate, and contain the resulting COVID-19 disease derived from limited knowledge. Screening for testing has been based on symptoms typically observed in inpatients, yet outpatient symptoms may differ. Classification and regression trees recursive partitioning created a decision tree classifying participants into laboratory-confirmed cases and non-cases. Demographic and symptom data from patients ages 18-87 years enrolled from March 29-June 8, 2020 were included. Presence or absence of SARS-CoV-2 was the target variable. Of 832 tested, 77 (9.3%) tested positive. Cases significantly more often reported diarrhea (12 percentage points (PP)), fever (15 PP), nausea/vomiting (9 PP), loss of taste/smell (52 PP), and contact with a COVID-19 case (54 PP), but less frequently reported sore throat (-27 PP). The 4-terminal node optimal tree had sensitivity of 69%, specificity of 78%, positive predictive value of 20%, negative predictive value of 97%, and AUC of 76%. Among those referred for testing, negative responses to two questions could classify about half (49%) of tested persons with low risk for SARS-CoV-2 and would save limited testing resources. Outpatient symptoms of COVID-19 appear to be broader than the inpatient syndrome.Initial supplies of anticipated COVID-19 vaccines may be limited and administration of first such available vaccines may need to be prioritized for essential workers, the most vulnerable, or those likely to have a robust response to vaccine. Another priority group could be those not previously infected. Those who screen out of testing may be less likely to have been infected by SARS-CoV-2 virus thus may be prioritized for vaccination when supplies are limited.
Keywords: COVID-19; SARS-CoV-2; classification trees; screening; symptoms.
Figures
Update of
-
Proposed Clinical Indicators for Efficient Screening and Testing for COVID-19 Infection from Classification and Regression Trees (CART) Analysis.medRxiv [Preprint]. 2020 May 14:2020.05.11.20097980. doi: 10.1101/2020.05.11.20097980. medRxiv. 2020. Update in: Hum Vaccin Immunother. 2021 Apr 3;17(4):1109-1112. doi: 10.1080/21645515.2020.1822135. PMID: 32511556 Free PMC article. Updated. Preprint.
Similar articles
-
Proposed Clinical Indicators for Efficient Screening and Testing for COVID-19 Infection from Classification and Regression Trees (CART) Analysis.medRxiv [Preprint]. 2020 May 14:2020.05.11.20097980. doi: 10.1101/2020.05.11.20097980. medRxiv. 2020. Update in: Hum Vaccin Immunother. 2021 Apr 3;17(4):1109-1112. doi: 10.1080/21645515.2020.1822135. PMID: 32511556 Free PMC article. Updated. Preprint.
-
COVID-19 symptoms at time of testing and association with positivity among outpatients tested for SARS-CoV-2.PLoS One. 2021 Dec 10;16(12):e0260879. doi: 10.1371/journal.pone.0260879. eCollection 2021. PLoS One. 2021. PMID: 34890441 Free PMC article.
-
Universal screening for SARS-CoV-2 infection: a rapid review.Cochrane Database Syst Rev. 2020 Sep 15;9(9):CD013718. doi: 10.1002/14651858.CD013718. Cochrane Database Syst Rev. 2020. PMID: 33502003 Free PMC article.
-
Effectiveness and cost-effectiveness of four different strategies for SARS-CoV-2 surveillance in the general population (CoV-Surv Study): a structured summary of a study protocol for a cluster-randomised, two-factorial controlled trial.Trials. 2021 Jan 8;22(1):39. doi: 10.1186/s13063-020-04982-z. Trials. 2021. PMID: 33419461 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2021 Feb 23;2(2):CD013665. doi: 10.1002/14651858.CD013665.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 20;5:CD013665. doi: 10.1002/14651858.CD013665.pub3. PMID: 33620086 Free PMC article. Updated.
Cited by
-
Differential expression of biomarkers in saliva related to SARS-CoV-2 infection in patients with mild, moderate and severe COVID-19.BMC Infect Dis. 2023 Sep 15;23(1):602. doi: 10.1186/s12879-023-08573-6. BMC Infect Dis. 2023. PMID: 37715121 Free PMC article.
-
Exploring the Clinical Utility of Gustatory Dysfunction (GD) as a Triage Symptom Prior to Reverse Transcription Polymerase Chain Reaction (RT-PCR) in the Diagnosis of COVID-19: A Meta-Analysis and Systematic Review.Life (Basel). 2021 Nov 29;11(12):1315. doi: 10.3390/life11121315. Life (Basel). 2021. PMID: 34947846 Free PMC article. Review.
-
Clinical Symptoms Among Ambulatory Patients Tested for SARS-CoV-2.Open Forum Infect Dis. 2020 Nov 26;8(1):ofaa576. doi: 10.1093/ofid/ofaa576. eCollection 2021 Jan. Open Forum Infect Dis. 2020. PMID: 33537361 Free PMC article.
-
Methodologies for the collection of parameters to estimate dust/soil ingestion for young children.Front Public Health. 2024 Jun 26;12:1357346. doi: 10.3389/fpubh.2024.1357346. eCollection 2024. Front Public Health. 2024. PMID: 38989126 Free PMC article.
-
Factors associated with COVID-19 viral and antibody test positivity and assessment of test concordance: a retrospective cohort study using electronic health records from the USA.BMJ Open. 2021 Oct 1;11(10):e051707. doi: 10.1136/bmjopen-2021-051707. BMJ Open. 2021. PMID: 34598988 Free PMC article.
References
-
- Ihle-Hansen H, Berge T, Tveita A, Rønning EJ, Ernø PE, Andersen EL, Wang CH, Tveit A, Myrstad M.. COVID-19: symptoms, course of illness and use of clinical scoring systems for the first 42 patients admitted to a Norwegian local hospital. Tidsskr Nor Laegeforen. 2020. doi:10.4045/tidsskr.20.0301. - DOI - PubMed
-
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD) Division of Viral Diseases . Symptoms of coronavirus. [accessed 2020 May 31].https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
-
- Lu X, Wang L, Sakthivel SK, Whitaker B, Murray J, Kamili S, Lynch B, Malapati L, Burke SA, Harcourt J. US CDC real-time reverse transcription pcr panel for detection of severe acute respiratory syndrome coronavirus 2. Emerging Infect Dis. 2020;26(8):8. doi:10.3201/eid2608.201246. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous