Lung megakaryocytes are immune modulatory cells
- PMID: 33079726
- PMCID: PMC7773372
- DOI: 10.1172/JCI137377
Lung megakaryocytes are immune modulatory cells
Abstract
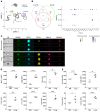
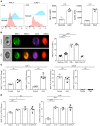
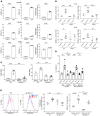
Although platelets are the cellular mediators of thrombosis, they are also immune cells. Platelets interact both directly and indirectly with immune cells, impacting their activation and differentiation, as well as all phases of the immune response. Megakaryocytes (Mks) are the cell source of circulating platelets, and until recently Mks were typically only considered bone marrow-resident (BM-resident) cells. However, platelet-producing Mks also reside in the lung, and lung Mks express greater levels of immune molecules compared with BM Mks. We therefore sought to define the immune functions of lung Mks. Using single-cell RNA sequencing of BM and lung myeloid-enriched cells, we found that lung Mks, which we term MkL, had gene expression patterns that are similar to antigen-presenting cells. This was confirmed using imaging and conventional flow cytometry. The immune phenotype of Mks was plastic and driven by the tissue immune environment, as evidenced by BM Mks having an MkL-like phenotype under the influence of pathogen receptor challenge and lung-associated immune molecules, such as IL-33. Our in vitro and in vivo assays demonstrated that MkL internalized and processed both antigenic proteins and bacterial pathogens. Furthermore, MkL induced CD4+ T cell activation in an MHC II-dependent manner both in vitro and in vivo. These data indicated that MkL had key immune regulatory roles dictated in part by the tissue environment.
Keywords: Hematology; Inflammation; Platelets.
Conflict of interest statement
Figures




Comment in
-
Location is everything when it comes to megakaryocyte function.J Clin Invest. 2021 Jan 4;131(1):e144964. doi: 10.1172/JCI144964. J Clin Invest. 2021. PMID: 33393510 Free PMC article.
References
-
- Aabo K, Hansen KB. Megakaryocytes in pulmonary blood vessels. I. Incidence at autopsy, clinicopathological relations especially to disseminated intravascular coagulation. Acta Pathol Microbiol Scand A. 1978;86(4):285–291. - PubMed
-
- Sharnoff JG, Kim ES. Pulmonary megakaryocyte studies in rabbits. AMA Arch Pathol. 1958;66(3):340–343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

