Homologous and heterologous re-challenge with Salmonella Typhi and Salmonella Paratyphi A in a randomised controlled human infection model
- PMID: 33079959
- PMCID: PMC7598925
- DOI: 10.1371/journal.pntd.0008783
Homologous and heterologous re-challenge with Salmonella Typhi and Salmonella Paratyphi A in a randomised controlled human infection model
Abstract
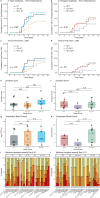
Enteric fever is a systemic infection caused by Salmonella Typhi or Paratyphi A. In many endemic areas, these serovars co-circulate and can cause multiple infection-episodes in childhood. Prior exposure is thought to confer partial, but incomplete, protection against subsequent attacks of enteric fever. Empirical data to support this hypothesis are limited, and there are few studies describing the occurrence of heterologous-protection between these closely related serovars. We performed a challenge-re-challenge study using a controlled human infection model (CHIM) to investigate the extent of infection-derived immunity to Salmonella Typhi or Paratyphi A infection. We recruited healthy volunteers into two groups: naïve volunteers with no prior exposure to Salmonella Typhi/Paratyphi A and volunteers previously-exposed to Salmonella Typhi or Paratyphi A in earlier CHIM studies. Within each group, participants were randomised 1:1 to oral challenge with either Salmonella Typhi (104 CFU) or Paratyphi A (103 CFU). The primary objective was to compare the attack rate between naïve and previously challenged individuals, defined as the proportion of participants per group meeting the diagnostic criteria of temperature of ≥38°C persisting for ≥12 hours and/or S. Typhi/Paratyphi bacteraemia up to day 14 post challenge. The attack-rate in participants who underwent homologous re-challenge with Salmonella Typhi was reduced compared with challenged naïve controls, although this reduction was not statistically significant (12/27[44%] vs. 12/19[63%]; Relative risk 0.70; 95% CI 0.41-1.21; p = 0.24). Homologous re-challenge with Salmonella Paratyphi A also resulted in a lower attack-rate than was seen in challenged naïve controls (3/12[25%] vs. 10/18[56%]; RR0.45; 95% CI 0.16-1.30; p = 0.14). Evidence of protection was supported by a post hoc analysis in which previous exposure was associated with an approximately 36% and 57% reduced risk of typhoid or paratyphoid disease respectively on re-challenge. Individuals who did not develop enteric fever on primary exposure were significantly more likely to be protected on re-challenge, compared with individuals who developed disease on primary exposure. Heterologous re-challenge with Salmonella Typhi or Salmonella Paratyphi A was not associated with a reduced attack rate following challenge. Within the context of the model, prior exposure was not associated with reduced disease severity, altered microbiological profile or boosting of humoral immune responses. We conclude that prior Salmonella Typhi and Paratyphi A exposure may confer partial but incomplete protection against subsequent infection, but with a comparable clinical and microbiological phenotype. There is no demonstrable cross-protection between these serovars, consistent with the co-circulation of Salmonella Typhi and Paratyphi A. Collectively, these data are consistent with surveillance and modelling studies that indicate multiple infections can occur in high transmission settings, supporting the need for vaccines to reduce the burden of disease in childhood and achieve disease control. Trial registration NCT02192008; clinicaltrials.gov.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: A.J.P chairs the UK Department of Health’s (DH) Joint Committee on Vaccination and Immunisation (JCVI) and the European Medicines Agency Scientific Advisory Group on Vaccines and is a member of the World Health Organization’s (WHO) Strategic Advisory Group of Experts. The views expressed in this manuscript are those of the authors and do not necessarily reflect the views of the JCVI, the DH, or the WHO. Author Vincenzo Cerundulo was unable to confirm his authorship contributions. On his behalf, the corresponding author has reported his contributions to the best of their knowledge.
Figures


References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

