Neuroretinal-Derived Caveolin-1 Promotes Endotoxin-Induced Inflammation in the Murine Retina
- PMID: 33079993
- PMCID: PMC7585394
- DOI: 10.1167/iovs.61.12.19
Neuroretinal-Derived Caveolin-1 Promotes Endotoxin-Induced Inflammation in the Murine Retina
Abstract
Purpose: The immune-privileged environment and complex organization of retinal tissue support the retina's essential role in visual function, yet confound inquiries into cell-specific inflammatory effects that lead to dysfunction and degeneration. Caveolin-1 (Cav1) is an integral membrane protein expressed in several retinal cell types and is implicated in immune regulation. However, whether Cav1 promotes or inhibits inflammatory processes in the retina (as well as in other tissues) remains unclear. Previously, we showed that global-Cav1 depletion resulted in reduced retinal inflammatory cytokine production but paradoxically elevated retinal immune cell infiltration. We hypothesized that these disparate responses are the result of differential cell-specific Cav1 functions in the retina.
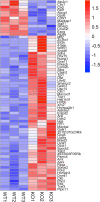
Methods: We used Cre/lox technology to deplete Cav1 specifically in the neural retinal (NR) compartment to clarify the role NR-specific Cav1 (NR-Cav1) in the retinal immune response to intravitreal inflammatory challenge induced by activation of Toll-like receptor-4 (TLR4). We used multiplex protein suspension array and flow cytometry to evaluate innate immune activation. Additionally, we used bioinformatics assessment of differentially expressed membrane-associated proteins to infer relationships between NR-Cav1 and immune response pathways.
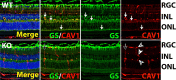
Results: NR-Cav1 depletion, which primarily affects Müller glia Cav1 expression, significantly altered immune response pathway regulators, decreased retinal inflammatory cytokine production, and reduced retinal immune cell infiltration in response to LPS-stimulated inflammatory induction.
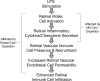
Conclusions: Cav1 expression in the NR compartment promotes the innate TLR4-mediated retinal tissue immune response. Additionally, we have identified novel potential immune modulators differentially expressed with NR-Cav1 depletion. This study further clarifies the role of NR-Cav1 in retinal inflammation.
Conflict of interest statement
Disclosure:
Figures




References
-
- Murakami Y, Ishikawa K, Nakao S, Sonoda KH. Innate immune response in retinal homeostasis and inflammatory disorders. Prog Retin Eye Res. 2019; 74: 100778. - PubMed
-
- Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol. 2003; 74: 179–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

