Association Between Early Treatment With Tocilizumab and Mortality Among Critically Ill Patients With COVID-19
- PMID: 33080002
- PMCID: PMC7577201
- DOI: 10.1001/jamainternmed.2020.6252
Association Between Early Treatment With Tocilizumab and Mortality Among Critically Ill Patients With COVID-19
Erratum in
-
Replacement of Nonauthor Collaborator Names.JAMA Intern Med. 2021 Apr 1;181(4):570. doi: 10.1001/jamainternmed.2021.0139. JAMA Intern Med. 2021. PMID: 33587090 Free PMC article. No abstract available.
Abstract
Importance: Therapies that improve survival in critically ill patients with coronavirus disease 2019 (COVID-19) are needed. Tocilizumab, a monoclonal antibody against the interleukin 6 receptor, may counteract the inflammatory cytokine release syndrome in patients with severe COVID-19 illness.
Objective: To test whether tocilizumab decreases mortality in this population.
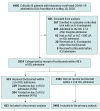
Design, setting, and participants: The data for this study were derived from a multicenter cohort study of 4485 adults with COVID-19 admitted to participating intensive care units (ICUs) at 68 hospitals across the US from March 4 to May 10, 2020. Critically ill adults with COVID-19 were categorized according to whether they received or did not receive tocilizumab in the first 2 days of admission to the ICU. Data were collected retrospectively until June 12, 2020. A Cox regression model with inverse probability weighting was used to adjust for confounding.
Exposures: Treatment with tocilizumab in the first 2 days of ICU admission.
Main outcomes and measures: Time to death, compared via hazard ratios (HRs), and 30-day mortality, compared via risk differences.
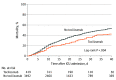
Results: Among the 3924 patients included in the analysis (2464 male [62.8%]; median age, 62 [interquartile range {IQR}, 52-71] years), 433 (11.0%) received tocilizumab in the first 2 days of ICU admission. Patients treated with tocilizumab were younger (median age, 58 [IQR, 48-65] vs 63 [IQR, 52-72] years) and had a higher prevalence of hypoxemia on ICU admission (205 of 433 [47.3%] vs 1322 of 3491 [37.9%] with mechanical ventilation and a ratio of partial pressure of arterial oxygen to fraction of inspired oxygen of <200 mm Hg) than patients not treated with tocilizumab. After applying inverse probability weighting, baseline and severity-of-illness characteristics were well balanced between groups. A total of 1544 patients (39.3%) died, including 125 (28.9%) treated with tocilizumab and 1419 (40.6%) not treated with tocilizumab. In the primary analysis, during a median follow-up of 27 (IQR, 14-37) days, patients treated with tocilizumab had a lower risk of death compared with those not treated with tocilizumab (HR, 0.71; 95% CI, 0.56-0.92). The estimated 30-day mortality was 27.5% (95% CI, 21.2%-33.8%) in the tocilizumab-treated patients and 37.1% (95% CI, 35.5%-38.7%) in the non-tocilizumab-treated patients (risk difference, 9.6%; 95% CI, 3.1%-16.0%).
Conclusions and relevance: Among critically ill patients with COVID-19 in this cohort study, the risk of in-hospital mortality in this study was lower in patients treated with tocilizumab in the first 2 days of ICU admission compared with patients whose treatment did not include early use of tocilizumab. However, the findings may be susceptible to unmeasured confounding, and further research from randomized clinical trials is needed.
Conflict of interest statement
Figures

Comment in
-
Time to Reassess Tocilizumab's Role in COVID-19 Pneumonia.JAMA Intern Med. 2021 Jan 1;181(1):12-15. doi: 10.1001/jamainternmed.2020.6557. JAMA Intern Med. 2021. PMID: 33079980 No abstract available.
References
-
- Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with COVID-19—preliminary report. Published online July 17, 2020. N Engl J Med. doi: 10.1101/2020.06.22.20137273 - DOI
Publication types
MeSH terms
Substances
Grants and funding
- K23 HL143053/HL/NHLBI NIH HHS/United States
- F32 HL149337/HL/NHLBI NIH HHS/United States
- R01 HL144566/HL/NHLBI NIH HHS/United States
- R01 DK125786/DK/NIDDK NIH HHS/United States
- P30 ES005022/ES/NIEHS NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- K12 HL138039/HL/NHLBI NIH HHS/United States
- R01 HL085757/HL/NHLBI NIH HHS/United States
- T32 HL087738/HL/NHLBI NIH HHS/United States
- P30 DK114857/DK/NIDDK NIH HHS/United States
- K08 GM134220/GM/NIGMS NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- K23 DK116967/DK/NIDDK NIH HHS/United States
- L30 HL149016/HL/NHLBI NIH HHS/United States
- F32 DC017342/DC/NIDCD NIH HHS/United States
- R01 HL153384/HL/NHLBI NIH HHS/United States
- R03 AG060179/AG/NIA NIH HHS/United States
- K23 DK120811/DK/NIDDK NIH HHS/United States
- UL1 TR002389/TR/NCATS NIH HHS/United States
- R37 AI102634/AI/NIAID NIH HHS/United States
- K23 HL130648/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

