Hydrogen Sulfide Oxidation by Sulfide Quinone Oxidoreductase
- PMID: 33080111
- PMCID: PMC7969369
- DOI: 10.1002/cbic.202000661
Hydrogen Sulfide Oxidation by Sulfide Quinone Oxidoreductase
Abstract
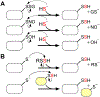
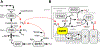
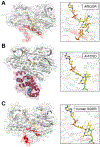
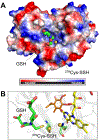
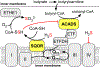
Hydrogen sulfide (H2 S) is an environmental toxin and a heritage of ancient microbial metabolism that has stimulated new interest following its discovery as a neuromodulator. While many physiological responses have been attributed to low H2 S levels, higher levels inhibit complex IV in the electron transport chain. To prevent respiratory poisoning, a dedicated set of enzymes that make up the mitochondrial sulfide oxidation pathway exists to clear H2 S. The committed step in this pathway is catalyzed by sulfide quinone oxidoreductase (SQOR), which couples sulfide oxidation to coenzyme Q10 reduction in the electron transport chain. The SQOR reaction prevents H2 S accumulation and generates highly reactive persulfide species as products; these can be further oxidized or can modify cysteine residues in proteins by persulfidation. Here, we review the kinetic and structural characteristics of human SQOR, and how its unconventional redox cofactor configuration and substrate promiscuity lead to sulfide clearance and potentially expand the signaling potential of H2 S. This dual role of SQOR makes it a promising target for H2 S-based therapeutics.
Keywords: flavins; metabolism; protein structure; redox chemistry; sulfides.
© 2020 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures
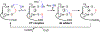
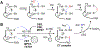
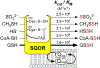
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

