Optic, trigeminal, and facial neuropathy related to anti-neurofascin 155 antibody
- PMID: 33080117
- PMCID: PMC7664262
- DOI: 10.1002/acn3.51220
Optic, trigeminal, and facial neuropathy related to anti-neurofascin 155 antibody
Abstract
Objective: To characterize the frequency and patterns of optic, trigeminal, and facial nerve involvement by neuroimaging and electrophysiology in IgG4 anti-neurofascin 155 antibody-positive (NF155+ ) chronic inflammatory demyelinating polyneuropathy (CIDP).
Methods: Thirteen IgG4 NF155+ CIDP patients with mean onset age of 34 years (11 men) were subjected to neurological examination, blink reflex, and visual-evoked potential (VEP) testing, and axial and/or coronal T2-weighted head magnetic resonance imaging (MRI).
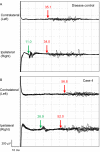
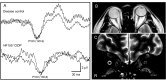
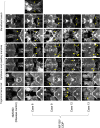
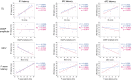
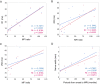
Results: Among 13 patients, facial sensory impairment, facial weakness, and apparent visual impairment were observed in three (23.1%), two (15.4%), and two (15.4%) patients, respectively. All 12 patients tested had blink reflex abnormalities: absent and/or delayed R1 in 11 (91.7%), and absent and/or delayed R2 in 10 (83.3%). R1 latencies had strong positive correlations with serum anti-NF155 antibody levels (r = 0.9, P ≤ 0.0001 on both sides) and distal and F wave latencies of the median and ulnar nerves. Absent and/or prolonged VEPs were observed in 10/13 (76.9%) patients and 17/26 (65.4%) eyes. On MRI, hypertrophy, and high signal intensity of trigeminal nerves were detected in 9/13 (69.2%) and 10/13 (76.9%) patients, respectively, whereas optic nerves were normal in all patients. The intra-orbital trigeminal nerve width on coronal sections showed a significant positive correlation with disease duration.
Interpretation: Subclinical demyelination frequently occurs in the optic, trigeminal, and facial nerves in IgG4 NF155+ CIDP, suggesting that both central and peripheral myelin structures of the cranial nerves are involved in this condition, whereas nerve hypertrophy only develops in myelinated peripheral nerve fibers.
© 2020 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Nothing to report.
Figures
References
-
- Dalakas MC. Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat Rev Neurol. 2011;7:507–517. - PubMed
-
- Joint Task Force of the EFNS and the PNS . European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripher. J. Peripher. Nerv. Syst. 2010;15:1–9. - PubMed
-
- Saperstein DS, Katz JS, Amato AA, Barohn RJ. Clinical spectrum of chronic acquired demyelinating polyneuropathies. Muscle Nerve 2001;24:311–324. - PubMed
-
- Guibord N, Chalk C, Wein F, et al. Trigeminal nerve hypertrophy in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology 1998;51:1459–1462. - PubMed
-
- Inoue H, Tsuboi Y, Tsugawa J, et al. Hypertrophic cranial nerve roots in CIDP. Neurology 2004;63:1481. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
