Visualizing HIF-1α mRNA in a Subpopulation of Bone Marrow-Derived Cells to Predict Retinal Neovascularization
- PMID: 33080135
- PMCID: PMC8121337
- DOI: 10.1021/acschembio.0c00662
Visualizing HIF-1α mRNA in a Subpopulation of Bone Marrow-Derived Cells to Predict Retinal Neovascularization
Abstract
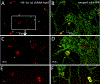
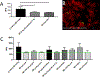
Bone marrow-derived progenitor cells and macrophages are known to migrate into the retina in response to inflammation and neovascularization. These migratory cells might play important regulatory roles in the pathogenesis of neovascularization, a common complication observed in diabetic retinopathy, retinopathy of prematurity, and retinal vein occlusion. Hypoxia-inducible factor 1α (HIF-1α) has been shown to contribute to the pathogenesis of retinal inflammation and neovascularization. However, contributions of monocyte-derived macrophages to neovascularization are largely unknown. We hypothesized that selective visualization of these microglia/macrophages could be a powerful method for predicting the onset of neovascularization and its progression at the molecular level. In this report, we describe the synthesis of a new hybrid nanoparticle to visualize HIF-1α mRNA selectively in microglia/macrophages in a mouse model of neovascularization. HIF-1α expression was confirmed in MRC-1 positive monocytes/macrophages as well as in CD4 positive T-cells and CD19 positive B-cells using single-cell RNA sequencing data analysis. The imaging probes (AS- or NS-shRNA-lipid) were synthesized by conjugating diacyl-lipids to short hairpin RNA with an antisense sequence complementary to HIF-1α mRNA and a fluorophore that is quenched by a black hole quencher. We believe that imaging mRNA selectively in tissue specific microglia/macrophages could be a powerful method for predicting the onset of neovascularization, its progression, and its response to therapy.
Conflict of interest statement
Figures






References
-
- Davies M, Eubanks J, Powers M. Microglia and macrophages are increased in response to ischemia-induced retinopathy in the mouse retina. Mol Vis 2006; 12(53–54): 467–77. - PubMed
-
- Gao X, Wang YS, Li XQ, et al. Macrophages promote vasculogenesis of retinal neovascularization in an oxygen-induced retinopathy model in mice. Cell Tissue Res 2016; 364(3): 599–610. - PubMed
-
- Kataoka K, Nishiguchi KM, Kaneko H, van Rooijen N, Kachi S, Terasaki H. The Roles of Vitreal Macrophages and Circulating Leukocytes in Retinal Neovascularization. Invest Ophth Vis Sci 2011; 52(3): 1431–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

