Dacron swab and PBS are acceptable alternatives to flocked swab and viral transport media for SARS-CoV-2
- PMID: 33080426
- PMCID: PMC7492856
- DOI: 10.1016/j.diagmicrobio.2020.115209
Dacron swab and PBS are acceptable alternatives to flocked swab and viral transport media for SARS-CoV-2
Abstract
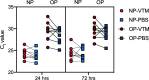
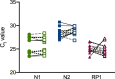
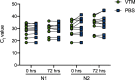
Nasopharyngeal flocked swabs placed in viral transport media (VTM) are the preferred collection methodology for respiratory virus testing. Due to the rapid depletion of available reagents and swabs, we have validated an alternative swab placed in phosphate-buffered saline (PBS) for use in respiratory virus testing in a SARS-CoV-2 real-time polymerase chain reaction assay and a multiplexed respiratory virus panel. We collected nasopharyngeal (NP) swabs and oropharyngeal (OP) swabs from 10 healthy volunteers. Flocked swabs were placed in VTM and alternative swabs in PBS. In this feasibility study, we show that NP collection is better for detection of human material than OP collection, as measured by significantly lower RNase P gene cycle threshold values, and that a Dacron polyester swab in PBS shows equivalent detection of SARS-CoV-2 and RSV to a flocked swab in VTM in contrived specimens. Diluted SARS-CoV-2-positive patient specimens are detectable for up to 72 h at 4 °C.
Keywords: 2019-nCOV; Alternative reagents; COVID-19; SARS-CoV-2.
Published by Elsevier Inc.
Figures
Similar articles
-
A Multiplex One-Step RT-qPCR Protocol to Detect SARS-CoV-2 in NP/OP Swabs and Saliva.Curr Protoc. 2021 May;1(5):e145. doi: 10.1002/cpz1.145. Curr Protoc. 2021. PMID: 34004070 Free PMC article.
-
Diagnostic Efficacy and Tolerability of Molded Plastic Nasopharyngeal Swab (FinSwab) Compared to Flocked Nylon Swab in Detection of SARS-CoV-2 and Other Respiratory Viruses.Microbiol Spectr. 2021 Oct 31;9(2):e0073621. doi: 10.1128/Spectrum.00736-21. Epub 2021 Oct 20. Microbiol Spectr. 2021. PMID: 34668741 Free PMC article.
-
Saliva is Comparable to Nasopharyngeal Swabs for Molecular Detection of SARS-CoV-2.Microbiol Spectr. 2021 Sep 3;9(1):e0016221. doi: 10.1128/Spectrum.00162-21. Epub 2021 Aug 18. Microbiol Spectr. 2021. PMID: 34406838 Free PMC article.
-
Performance of Saliva, Oropharyngeal Swabs, and Nasal Swabs for SARS-CoV-2 Molecular Detection: a Systematic Review and Meta-analysis.J Clin Microbiol. 2021 Apr 20;59(5):e02881-20. doi: 10.1128/JCM.02881-20. Print 2021 Apr 20. J Clin Microbiol. 2021. PMID: 33504593 Free PMC article.
-
Detection profile of SARS-CoV-2 using RT-PCR in different types of clinical specimens: A systematic review and meta-analysis.J Med Virol. 2021 Feb;93(2):719-725. doi: 10.1002/jmv.26349. Epub 2020 Aug 2. J Med Virol. 2021. PMID: 32706393 Free PMC article.
Cited by
-
Performance of nasopharyngeal swab and saliva in detecting Delta and Omicron SARS-CoV-2 variants.J Med Virol. 2022 Oct;94(10):4704-4711. doi: 10.1002/jmv.27898. Epub 2022 Jun 8. J Med Virol. 2022. PMID: 35642439 Free PMC article.
-
Assessment of SARS-CoV-2 viral loads in combined nasal-and-throat swabs collected from COVID-19 individuals under the Universal Community Testing Programme in Hong Kong.J Virol Methods. 2022 Feb;300:114396. doi: 10.1016/j.jviromet.2021.114396. Epub 2021 Nov 29. J Virol Methods. 2022. PMID: 34856306 Free PMC article.
-
Evaluation of a bioaerosol sampler for indoor environmental surveillance of Severe Acute Respiratory Syndrome Coronavirus 2.PLoS One. 2021 Nov 15;16(11):e0257689. doi: 10.1371/journal.pone.0257689. eCollection 2021. PLoS One. 2021. PMID: 34780482 Free PMC article.
-
Clinical Application of the Novel Cell-Based Biosensor for the Ultra-Rapid Detection of the SARS-CoV-2 S1 Spike Protein Antigen: A Practical Approach.Biosensors (Basel). 2021 Jul 6;11(7):224. doi: 10.3390/bios11070224. Biosensors (Basel). 2021. PMID: 34356695 Free PMC article.
References
-
- ASM ASM expresses concern about coronavirus test reagent shortages. 2020, March 10. https://www.asm.org/Articles/Policy/2020/March/ASM-Expresses-Concern-abo... Available from:
-
- CDC Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019 (COVID-19) 2020, April 14. https://www.cdc.gov/coronavirus/2019-ncov/lab/lab-biosafety-guidelines.html Available from:
-
- FDA Letter of authorization. 2020, March 15. https://www.fda.gov/media/134919/download Available from:
-
- Mitchell S., St. George K., Rhoads D., Butler-Wu S., Dharmarha V., McNult P. Verification procedure for commercial tests with Emergency Use Authorization for the detection of SARS-CoV-2 RNA American Society of Microbiology. 2020, April 3. https://asm.org/ASM/media/Protocol-Images/ASM_EUA_verification_040220_FI... Available from: - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

