Identification and Tracking of Antiviral Drug Combinations
- PMID: 33080984
- PMCID: PMC7589631
- DOI: 10.3390/v12101178
Identification and Tracking of Antiviral Drug Combinations
Abstract
Combination therapies have become a standard for the treatment for HIV and hepatitis C virus (HCV) infections. They are advantageous over monotherapies due to better efficacy, reduced toxicity, as well as the ability to prevent the development of resistant viral strains and to treat viral co-infections. Here, we identify new synergistic combinations against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), echovirus 1 (EV1), hepatitis C virus (HCV) and human immunodeficiency virus 1 (HIV-1) in vitro. We observed synergistic activity of nelfinavir with convalescent serum and with purified neutralizing antibody 23G7 against SARS-CoV-2 in human lung epithelial Calu-3 cells. We also demonstrated synergistic activity of nelfinavir with EIDD-2801 or remdesivir in Calu-3 cells. In addition, we showed synergistic activity of vemurafenib with emetine, homoharringtonine, anisomycin, or cycloheximide against EV1 infection in human lung epithelial A549 cells. We also found that combinations of sofosbuvir with brequinar or niclosamide are synergistic against HCV infection in hepatocyte-derived Huh-7.5 cells, and that combinations of monensin with lamivudine or tenofovir are synergistic against HIV-1 infection in human cervical TZM-bl cells. These results indicate that synergy is achieved when a virus-directed antiviral is combined with another virus- or host-directed agent. Finally, we present an online resource that summarizes novel and known antiviral drug combinations and their developmental status.
Keywords: antiviral drug combinations; antivirals; broad-spectrum antivirals; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

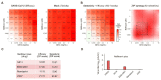




References
-
- DALYs G.B.D., Collaborators H. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1260–1344. doi: 10.1016/S0140-6736(17)32130-X. - DOI - PMC - PubMed
-
- Disease G.B.D., Injury I., Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

