Crosstalk of Hedgehog and mTORC1 Pathways
- PMID: 33081032
- PMCID: PMC7603200
- DOI: 10.3390/cells9102316
Crosstalk of Hedgehog and mTORC1 Pathways
Abstract
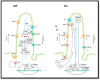
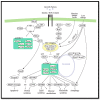
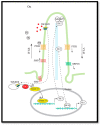
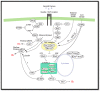
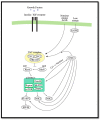
Hedgehog (Hh) signaling and mTOR signaling, essential for embryonic development and cellular metabolism, are both coordinated by the primary cilium. Observations from cancer cells strongly indicate crosstalk between Hh and mTOR signaling. This hypothesis is supported by several studies: Evidence points to a TGFβ-mediated crosstalk; Increased PI3K/AKT/mTOR activity leads to increased Hh signaling through regulation of the GLI transcription factors; increased Hh signaling regulates mTORC1 activity positively by upregulating NKX2.2, leading to downregulation of negative mTOR regulators; GSK3 and AMPK are, as members of both signaling pathways, potentially important links between Hh and mTORC1 signaling; The kinase DYRK2 regulates Hh positively and mTORC1 signaling negatively. In contrast, both positive and negative regulation of Hh has been observed for DYRK1A and DYRK1B, which both regulate mTORC1 signaling positively. Based on crosstalk observed between cilia, Hh, and mTORC1, we suggest that the interaction between Hh and mTORC1 is more widespread than it appears from our current knowledge. Although many studies focusing on crosstalk have been carried out, contradictory observations appear and the interplay involving multiple partners is far from solved.
Keywords: 4E-BP1; GLI1; GLI2; GLI3; Mammalian target of rapamycin; S6K; TSC; autophagy; eIF4E; primary cilia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
TSC1 and TSC2 regulate cilia length and canonical Hedgehog signaling via different mechanisms.Cell Mol Life Sci. 2018 Jul;75(14):2663-2680. doi: 10.1007/s00018-018-2761-8. Epub 2018 Feb 2. Cell Mol Life Sci. 2018. PMID: 29396625 Free PMC article.
-
mTORC1-Mediated Inhibition of 4EBP1 Is Essential for Hedgehog Signaling-Driven Translation and Medulloblastoma.Dev Cell. 2017 Dec 18;43(6):673-688.e5. doi: 10.1016/j.devcel.2017.10.011. Epub 2017 Nov 2. Dev Cell. 2017. PMID: 29103956 Free PMC article.
-
mTORC1 hampers Hedgehog signaling in Tsc2 deficient cells.Life Sci Alliance. 2024 Aug 26;7(11):e202302419. doi: 10.26508/lsa.202302419. Print 2024 Nov. Life Sci Alliance. 2024. PMID: 39187374 Free PMC article.
-
Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship.Adv Biol Regul. 2019 May;72:51-62. doi: 10.1016/j.jbior.2019.03.003. Epub 2019 Apr 11. Adv Biol Regul. 2019. PMID: 31010692 Review.
-
Discrete signaling mechanisms of mTORC1 and mTORC2: Connected yet apart in cellular and molecular aspects.Adv Biol Regul. 2017 May;64:39-48. doi: 10.1016/j.jbior.2016.12.001. Epub 2017 Jan 4. Adv Biol Regul. 2017. PMID: 28189457 Review.
Cited by
-
Look who's TORking: mTOR-mediated integration of cell status and external signals during limb development and endochondral bone growth.Front Cell Dev Biol. 2023 Apr 19;11:1153473. doi: 10.3389/fcell.2023.1153473. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37152288 Free PMC article. Review.
-
Crosstalk between the mTOR pathway and primary cilia in human diseases.Curr Top Dev Biol. 2023;155:1-37. doi: 10.1016/bs.ctdb.2023.09.004. Epub 2023 Nov 4. Curr Top Dev Biol. 2023. PMID: 38043949 Free PMC article. Review.
-
The Role of Cytokines in Activation of Tumour-promoting Pathways and Emergence of Cancer Drug Resistance.Curr Top Med Chem. 2024;24(6):523-540. doi: 10.2174/0115680266284527240118041129. Curr Top Med Chem. 2024. PMID: 38258788 Review.
-
PDE4 inhibitor rolipram represses hedgehog signaling via ubiquitin-mediated proteolysis of GLI transcription factors to regress breast cancer.J Biol Chem. 2025 Mar;301(3):108239. doi: 10.1016/j.jbc.2025.108239. Epub 2025 Jan 27. J Biol Chem. 2025. PMID: 39880092 Free PMC article.
-
Autophagy and the primary cilium in cell metabolism: What's upstream?Front Cell Dev Biol. 2022 Nov 9;10:1046248. doi: 10.3389/fcell.2022.1046248. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36438551 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

