Human Isogenic Cell Line Models for Neutrophils and Myeloid-Derived Suppressor Cells
- PMID: 33081041
- PMCID: PMC7590135
- DOI: 10.3390/ijms21207709
Human Isogenic Cell Line Models for Neutrophils and Myeloid-Derived Suppressor Cells
Abstract
Neutrophils with immunosuppressive activity are polymorphonuclear myeloid-derived suppressor cells (MDSCs) and may contribute to the resistance to cancer immunotherapy. A major gap for understanding and targeting these cells is the paucity of cell line models with cardinal features of human immunosuppressive neutrophils and their normal counterparts, especially in an isogenic manner. To address this issue, we employ the human promyelocytic cell line HL60 and use DMSO and cytokines (granulocyte macrophage-colony stimulating factor (GM-CSF) and interleukin 6 (IL6)) to induce the formation of either neutrophils or MDSCs. The induced MDSCs are CD11b+ CD33+ HLA-DR-/low and are heterogeneous for CD15 and CD14 expression. The induced MDSCs abrogate IL2 production and activation-induced cell death of the human T cell line Jurkat stimulated by CD3/CD28 antibodies, whereas the induced neutrophils enhance IL2 production from Jurkat cells. The induced MDSCs upregulate the expression of C/EBPβ, STAT3, VEGFR1, FATP2 and S100A8. Lastly, the immunosuppressive activity of the induced MDSCs is inhibited by all-trans retinoic acid and STAT3 inhibitor BP-1-102 through cellular differentiation and dedifferentiation mechanisms, respectively. Together, our study establishes a human isogenic cell line system for neutrophils and MDSCs and this system is expected to facilitate future studies on the biology and therapeutics of human immunosuppressive neutrophils.
Keywords: GM-CSF; HL60; IL6; Jurkat; PMN-MDSC; STAT3 inhibitor; all-trans retinoic acid; myeloid-derived suppressor cell; neutrophil.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

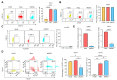
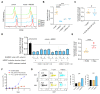
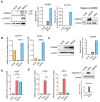
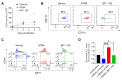
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

