A Facile Approach for Rapid Prototyping of Microneedle Molds, Microwells and Micro-Through-Holes in Various Substrate Materials Using CO2 Laser Drilling
- PMID: 33081055
- PMCID: PMC7603185
- DOI: 10.3390/biomedicines8100427
A Facile Approach for Rapid Prototyping of Microneedle Molds, Microwells and Micro-Through-Holes in Various Substrate Materials Using CO2 Laser Drilling
Abstract
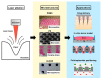
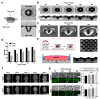
CO2 laser manufacturing has served as an enabling and reliable tool for rapid and cost-effective microfabrication over the past few decades. While a wide range of industrial and biological applications have been studied, the choice of materials fabricated across various laser parameters and systems is often confounded by their complex combinations. We herein presented a unified procedure performed using percussion CO2 laser drilling with a range of laser parameters, substrate materials and various generated microstructures, enabling a variety of downstream tissue/cellular-based applications. Emphasis is placed on delineating the laser drilling effect on different biocompatible materials and proof-of-concept utilities. First, a polydimethylsiloxane (PDMS) microneedle (MN) array mold is fabricated to generate dissolvable polyvinylpyrrolidone/polyvinyl alcohol (PVP/PVA) MNs for transdermal drug delivery. Second, polystyrene (PS) microwells are optimized in a compact array for the formation of size-controlled multicellular tumor spheroids (MCTSs). Third, coverglass is perforated to form a microaperture that can be used to trap/position cells/spheroids. Fourth, the creation of through-holes in PS is validated as an accessible method to create channels that facilitate medium exchange in hanging drop arrays and as a conducive tool for the growth and drug screenings of MCTSs.
Keywords: CO2 laser; hanging drops; microneedle; microwells; multicellular tumor spheroids; rapid prototyping.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
A fabrication method of microneedle molds with controlled microstructures.Mater Sci Eng C Mater Biol Appl. 2016 Aug 1;65:135-42. doi: 10.1016/j.msec.2016.03.097. Epub 2016 Apr 13. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 27157736
-
Design, analysis and fabrication of solid polymer microneedle patch using CO2 laser and polymer molding.Drug Deliv Transl Res. 2023 Jun;13(6):1813-1827. doi: 10.1007/s13346-023-01296-w. Epub 2023 Feb 20. Drug Deliv Transl Res. 2023. PMID: 36807879
-
Rapid prototyping of concave microwells for the formation of 3D multicellular cancer aggregates for drug screening.Adv Healthc Mater. 2014 Apr;3(4):609-16. doi: 10.1002/adhm.201300151. Epub 2013 Aug 27. Adv Healthc Mater. 2014. PMID: 23983140 Free PMC article.
-
Microneedle, bio-microneedle and bio-inspired microneedle: A review.J Control Release. 2017 Apr 10;251:11-23. doi: 10.1016/j.jconrel.2017.02.011. Epub 2017 Feb 16. J Control Release. 2017. PMID: 28215667 Review.
-
Potential of microneedle-assisted micro-particle delivery by gene guns: a review.Drug Deliv. 2014 Dec;21(8):571-87. doi: 10.3109/10717544.2013.864345. Epub 2013 Dec 9. Drug Deliv. 2014. PMID: 24313864 Review.
Cited by
-
A Preliminary Investigation of Embedding In Vitro HepaRG Spheroids into Recombinant Human Collagen Type I for the Promotion of Liver Differentiation.Polymers (Basel). 2022 May 9;14(9):1923. doi: 10.3390/polym14091923. Polymers (Basel). 2022. PMID: 35567092 Free PMC article.
-
An overview of microneedle applications, materials, and fabrication methods.Beilstein J Nanotechnol. 2021 Sep 13;12:1034-1046. doi: 10.3762/bjnano.12.77. eCollection 2021. Beilstein J Nanotechnol. 2021. PMID: 34621614 Free PMC article. Review.
-
Microneedle Sensors for Point-of-Care Diagnostics.Adv Sci (Weinh). 2024 Mar;11(12):e2306560. doi: 10.1002/advs.202306560. Epub 2024 Jan 15. Adv Sci (Weinh). 2024. PMID: 38225744 Free PMC article. Review.
-
Poly(2-Hydroxyethyl Methacrylate) Hydrogel-Based Microneedles for Bioactive Release.Bioengineering (Basel). 2024 Jun 25;11(7):649. doi: 10.3390/bioengineering11070649. Bioengineering (Basel). 2024. PMID: 39061731 Free PMC article.
-
Establishment of Colorectal Cancer Organoids in Microfluidic-Based System.Micromachines (Basel). 2021 Apr 28;12(5):497. doi: 10.3390/mi12050497. Micromachines (Basel). 2021. PMID: 33924829 Free PMC article.
References
-
- Wu W.I., Rezai P., Hsu H.H., Selvaganapathy P.R. Materials and methods for the microfabrication of microfluidic biomedical devices. In: Li X.J., Zhou Y., editors. Microfluidic Devices for Biomedical Applications. Woodhead Publishing; Cambridge, UK: 2013. pp. 3–62.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

