Allium subhirsutum L. as a Potential Source of Antioxidant and Anticancer Bioactive Molecules: HR-LCMS Phytochemical Profiling, In Vitro and In Vivo Pharmacological Study
- PMID: 33081189
- PMCID: PMC7602730
- DOI: 10.3390/antiox9101003
Allium subhirsutum L. as a Potential Source of Antioxidant and Anticancer Bioactive Molecules: HR-LCMS Phytochemical Profiling, In Vitro and In Vivo Pharmacological Study
Abstract
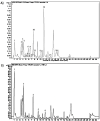
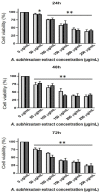
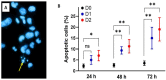
This study investigated Allium subhirsutum L. (AS) anticancer and antioxidant effects and inhibition of tumor angiogenesis in a murine model of skeletal metastases due to inoculation of Walker 256/B cells. Phytochemical composition of AS extract (ASE) was studied by High Resolution-Liquid Chromatography Mass Spectroscopy (HR-LCMS). Total phenolic and flavonoid contents (TPC and TFC) were determined. In vitro, the antioxidant properties were evaluated by reducing power and antiradical activity against DPPH. Cancer cells' proliferation, apoptosis, metastatic development and angiogenesis were evaluated using Walker 256/B and MatLyLu cells. The p-coumaric acid was the major phenolic acid (1700 µg/g extract). ASE showed high levels of TPC and TFC and proved potent antioxidant effects. ASE inhibited Walker 256/B and MatLyLu cells' proliferation (Half-maximal inhibitory concentration: IC50 ≃ 150 µg/mL) and induced apoptosis. In silico and in vivo assays confirmed these findings. ASE effectively acts as a chemo-preventive compound, induces apoptosis and attenuates angiogenesis and osteolytic metastases due to Walker 256/B malignant cells.
Keywords: Allium subhirsutum; angiogenesis; anticancer; antioxidant; apoptosis; bone metastases; cell proliferation; phytochemistry.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y., et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. - DOI - PMC - PubMed
-
- Zambrowski J.J. Costs of cancer care. Rev. Prat. 2016;66:489–493. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

