IgE Antibodies against Cancer: Efficacy and Safety
- PMID: 33081206
- PMCID: PMC7709114
- DOI: 10.3390/antib9040055
IgE Antibodies against Cancer: Efficacy and Safety
Abstract
Immunoglobulin E (IgE) antibodies are well known for their role in allergic diseases and for contributions to antiparasitic immune responses. Properties of this antibody class that mediate powerful effector functions may be redirected for the treatment of solid tumours. This has led to the rise of a new class of therapeutic antibodies to complement the armamentarium of approved tumour targeting antibodies, which to date are all IgG class. The perceived risk of type I hypersensitivity reactions following administration of IgE has necessitated particular consideration in the development of these therapeutic agents. Here, we bring together the properties of IgE antibodies pivotal to the hypothesis for superior antitumour activity compared to IgG, observations of in vitro and in vivo efficacy and mechanisms of action, and a focus on the safety considerations for this novel class of therapeutic agent. These include in vitro studies of potential hypersensitivity, selection of and observations from appropriate in vivo animal models and possible implications of the high degree of glycosylation of IgE. We also discuss the use of ex vivo predictive and monitoring clinical tools, as well as the risk mitigation steps employed in, and the preliminary outcomes from, the first-in-human clinical trial of a candidate anticancer IgE therapeutic.
Keywords: AllergoOncology; IgE; anaphylaxis; antibodies; basophil activation test (BAT); cancer; immunotherapy; in vivo models; safety; type I hypersensitivity.
Conflict of interest statement
V.F.C., J.F.S. and S.N.K. are founders and shareholders of Epsilogen Ltd.; H.J.B. and H.S.S. are employed through a fund provided by Epsilogen Ltd. All other authors declare no conflicts of interest. The authors are solely responsible for decision to publish, and preparation of the manuscript. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Figures

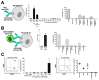


References
-
- Wulaningsih W., Holmberg L., Garmo H., Karagiannis S.N., Ahlstedt S., Malmstrom H., Lambe M., Hammar N., Walldius G., Jungner I., et al. Investigating the association between allergen-specific immunoglobulin E, cancer risk and survival. Oncoimmunology. 2016;5:e1154250. doi: 10.1080/2162402X.2016.1154250. - DOI - PMC - PubMed
-
- Schwartzbaum J., Seweryn M., Holloman C., Harris R., Handelman S.K., Rempala G.A., Huang R.P., Burkholder B., Brandemihl A., Kallberg H., et al. Association between Prediagnostic Allergy-Related Serum Cytokines and Glioma. PLoS ONE. 2015;10:e0137503. doi: 10.1371/journal.pone.0137503. - DOI - PMC - PubMed
Publication types
Grants and funding
- -/Guy's and St Thomas's Foundation Trust Charity Melanoma Special Fund
- IS-BRC-1215-20006/National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) based at Guy's and St Thomas' NHS Foundation Trust and King's College London
- 147; KCL-BCN-Q3/BBC_/Breast Cancer Now/United Kingdom
- C604/A25135/Cancer Research UK King's Health Partners Centre at King's College London
- C10355/A15587/CRUK//NIHR in England/DoH for Scotland, Wales and Northern Ireland Experimental Cancer Medicine Centre
LinkOut - more resources
Full Text Sources
Other Literature Sources

