A Review of Molecular Imaging of Glutamate Receptors
- PMID: 33081223
- PMCID: PMC7587586
- DOI: 10.3390/molecules25204749
A Review of Molecular Imaging of Glutamate Receptors
Abstract
Molecular imaging with positron emission tomography (PET) and single photon emission computed tomography (SPECT) is a well-established and important in vivo technique to evaluate fundamental biological processes and unravel the role of neurotransmitter receptors in various neuropsychiatric disorders. Specific ligands are available for PET/SPECT studies of dopamine, serotonin, and opiate receptors, but corresponding development of radiotracers for receptors of glutamate, the main excitatory neurotransmitter in mammalian brain, has lagged behind. This state of affairs has persisted despite the central importance of glutamate neurotransmission in brain physiology and in disorders such as stroke, epilepsy, schizophrenia, and neurodegenerative diseases. Recent years have seen extensive efforts to develop useful ligands for molecular imaging of subtypes of the ionotropic (N-methyl-D-aspartate (NMDA), kainate, and AMPA/quisqualate receptors) and metabotropic glutamate receptors (types I, II, and III mGluRs). We now review the state of development of radioligands for glutamate receptor imaging, placing main emphasis on the suitability of available ligands for reliable in vivo applications. We give a brief account of the radiosynthetic approach for selected molecules. In general, with the exception of ligands for the GluN2B subunit of NMDA receptors, there has been little success in developing radiotracers for imaging ionotropic glutamate receptors; failure of ligands for the PCP/MK801 binding site in vivo doubtless relates their dependence on the open, unblocked state of the ion channel. Many AMPA and kainite receptor ligands with good binding properties in vitro have failed to give measurable specific binding in the living brain. This may reflect the challenge of developing brain-penetrating ligands for amino acid receptors, compounded by conformational differences in vivo. The situation is better with respect to mGluR imaging, particularly for the mGluR5 subtype. Several successful PET ligands serve for investigations of mGluRs in conditions such as schizophrenia, depression, substance abuse and aging. Considering the centrality and diversity of glutamatergic signaling in brain function, we have relatively few selective and sensitive tools for molecular imaging of ionotropic and metabotropic glutamate receptors. Further radiopharmaceutical research targeting specific subtypes and subunits of the glutamate receptors may yet open up new investigational vistas with broad applications in basic and clinical research.
Keywords: glutamate receptors; positron emission tomography; radioligands; single photon emission computed tomography.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
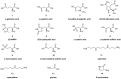
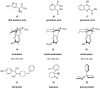


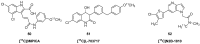
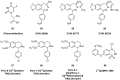

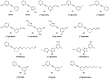
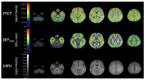
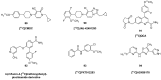
Similar articles
-
PET and SPECT tracers for glutamate receptors.Drug Discov Today. 2013 Feb;18(3-4):173-84. doi: 10.1016/j.drudis.2012.10.004. Epub 2012 Oct 22. Drug Discov Today. 2013. PMID: 23092894 Review.
-
Development of PET and SPECT probes for glutamate receptors.ScientificWorldJournal. 2015;2015:716514. doi: 10.1155/2015/716514. Epub 2015 Mar 22. ScientificWorldJournal. 2015. PMID: 25874256 Free PMC article. Review.
-
Ionotropic and metabotropic glutamate receptor structure and pharmacology.Psychopharmacology (Berl). 2005 Apr;179(1):4-29. doi: 10.1007/s00213-005-2200-z. Epub 2005 Feb 25. Psychopharmacology (Berl). 2005. PMID: 15731895 Review.
-
Imaging the glutamate system in humans: relevance to drug discovery for schizophrenia.Curr Pharm Des. 2009;15(22):2594-602. doi: 10.2174/138161209788957438. Curr Pharm Des. 2009. PMID: 19689330 Review.
-
[Role of excitatory amino acids in neuropathology].Medicina (B Aires). 1995;55(4):355-65. Medicina (B Aires). 1995. PMID: 8728878 Review. Spanish.
Cited by
-
Postsynaptic Proteins at Excitatory Synapses in the Brain-Relationship with Depressive Disorders.Int J Mol Sci. 2022 Sep 28;23(19):11423. doi: 10.3390/ijms231911423. Int J Mol Sci. 2022. PMID: 36232725 Free PMC article. Review.
-
Presurgical Executive Functioning in Low-Grade Glioma Patients Cannot Be Topographically Mapped.Cancers (Basel). 2023 Jan 28;15(3):807. doi: 10.3390/cancers15030807. Cancers (Basel). 2023. PMID: 36765764 Free PMC article.
-
Unraveling the nexus of age, epilepsy, and mitochondria: exploring the dynamics of cellular energy and excitability.Front Pharmacol. 2024 Sep 5;15:1469053. doi: 10.3389/fphar.2024.1469053. eCollection 2024. Front Pharmacol. 2024. PMID: 39309002 Free PMC article. Review.
-
Utility of SPECT Functional Neuroimaging of Pain.Front Psychiatry. 2021 Jul 29;12:705242. doi: 10.3389/fpsyt.2021.705242. eCollection 2021. Front Psychiatry. 2021. PMID: 34393862 Free PMC article.
-
Spider and Wasp Acylpolyamines: Venom Components and Versatile Pharmacological Leads, Probes, and Insecticidal Agents.Toxins (Basel). 2024 May 21;16(6):234. doi: 10.3390/toxins16060234. Toxins (Basel). 2024. PMID: 38922129 Free PMC article. Review.
References
-
- Kerkut G.A., Walker R.J. The effect of L-glutamate, acetylcholine and gamma-aminobutyric acid on the miniature end-plate potentials and contractures of the coxal muscles of the cockroach, Periplaneta Americana. Comp. Biochem. Physiol. 1966;17:435–454. doi: 10.1016/0010-406X(66)90579-2. - DOI - PubMed
-
- Schousboe A. Transport and metabolism of glutamate and GABA in neurons and glial cells. In: Smythies J.R., Bradley R.J., editors. International Review of Neurobiolog. Volume 22. Academic Press; Cambridge, MA, USA: 1981. pp. 1–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

