The Role of Zinc in Copper Homeostasis of Aspergillus fumigatus
- PMID: 33081273
- PMCID: PMC7593903
- DOI: 10.3390/ijms21207665
The Role of Zinc in Copper Homeostasis of Aspergillus fumigatus
Abstract
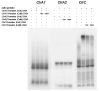
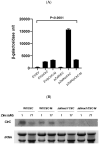
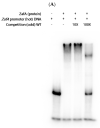
Copper is an essential metal ion that performs many physiological functions in living organisms. Deletion of Afmac1, which is a copper-responsive transcriptional activator in A. fumigatus, results in a growth defect on aspergillus minimal medium (AMM). Interestingly, we found that zinc starvation suppressed the growth defect of the Δafmac1 strain on AMM. In addition, the growth defect of the Δafmac1 strain was recovered by copper supplementation or introduction of the CtrC gene into the Δafmac1 strain. However, chelation of copper by addition of BCS to AMM failed to recover the growth defect of the Δafmac1 strain. Through Northern blot analysis, we found that zinc starvation upregulated CtrC and CtrA2, which encode membrane copper transporters. Interestingly, we found that the conserved ZafA binding motif 5'-CAA(G)GGT-3' was present in the upstream region of CtrC and CtrA2 and that mutation of the binding motif led to failure of ZafA binding to the upstream region of CtrC and upregulation of CtrC expression under zinc starvation. Furthermore, the binding activity of ZafA to the upstream region of CtrC was inversely proportional to the zinc concentration, and copper inhibited the binding of ZafA to the upstream region of CtrC under a low zinc concentration. Taken together, these results suggest that ZafA upregulates copper metabolism by binding to the ZafA binding motif in the CtrC promoter region under low zinc concentration, thus regulating copper homeostasis. Furthermore, we found that copper and zinc interact in cells to maintain metal homeostasis.
Keywords: Aspergillus fumigatus; CtrC; ZafA; copper; zinc.
Conflict of interest statement
We declare that we have no competing interests.
Figures





References
-
- Porcheron G., Garénaux A., Proulx J., Sabri M., Dozois C.M. Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: Correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front. Cell. Infect. Microbiol. 2013 doi: 10.3389/fcimb.2013.00090. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

