Conjugation of Native-Like HIV-1 Envelope Trimers onto Liposomes Using EDC/Sulfo-NHS Chemistry: Requirements and Limitations
- PMID: 33081278
- PMCID: PMC7589475
- DOI: 10.3390/pharmaceutics12100979
Conjugation of Native-Like HIV-1 Envelope Trimers onto Liposomes Using EDC/Sulfo-NHS Chemistry: Requirements and Limitations
Abstract
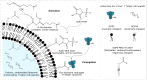
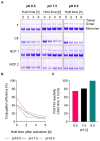
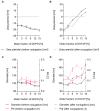
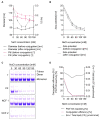
The display of native-like human immunodeficiency virus type 1 envelope (HIV-1 Env) trimers on liposomes has gained wide attention over the last few years. Currently, available methods have enabled the preparation of Env-liposome conjugates of unprecedented quality. However, these protocols require the Env trimer to be tagged and/or to carry a specific functional group. For this reason, we have investigated N-(3-Dimethylaminopropyl)-N'-ethylcarbodiimide/N-Hydroxysulfosuccinimide (EDC/Sulfo-NHS) chemistry for its potential to covalently conjugate tag-free, non-functionalized native-like Env trimers onto the surface of carboxyl-functionalized liposomes. The preservation of the liposome's physical integrity and the immunogen's conformation required a fine-tuned two-step approach based on the controlled use of β-mercaptoethanol. The display of Env trimers was strictly limited to activated liposomes of positive charge, i.e., liposomes with a positive zeta potential that carry amine-reactive Sulfo-NHS esters on their surface. In agreement with that, conjugation was found to be highly ionic strength- and pH-dependent. Overall, we have identified electrostatic pre-concentration (i.e., close proximity between negatively charged Env trimers and positively charged liposomes established through electrostatic attraction) to be crucial for conjugation reactions to proceed. The present study highlights the requirements and limitations of potentially scalable EDC/Sulfo-NHS-based approaches and represents a solid basis for further research into the controlled conjugation of tag-free, non-functionalized native-like Env trimers on the surface of liposomes, and other nanoparticles.
Keywords: EDC/Sulfo-NHS; HIV-1; covalent conjugation; intrastructural help; liposomes; native-like Env trimers; particulate display; pre-concentration; protein-liposome conjugates; tag-free conjugation; vaccines.
Conflict of interest statement
The authors declare no conflict of interest. E.S. and A.W. are employees of Polymun Scientific Immunbiologische Forschung GmbH and they declared no competing financial interests.
Figures




Similar articles
-
Design and Functional Characterization of HIV-1 Envelope Protein-Coupled T Helper Liposomes.Pharmaceutics. 2022 Jun 30;14(7):1385. doi: 10.3390/pharmaceutics14071385. Pharmaceutics. 2022. PMID: 35890282 Free PMC article.
-
Covalent coupling of HIV-1 glycoprotein trimers to biodegradable calcium phosphate nanoparticles via genetically encoded aldehyde-tags.Acta Biomater. 2022 Mar 1;140:586-600. doi: 10.1016/j.actbio.2021.12.022. Epub 2021 Dec 27. Acta Biomater. 2022. PMID: 34968725
-
Modulation of immune responses to liposomal vaccines by intrastructural help.Eur J Pharm Biopharm. 2023 Nov;192:112-125. doi: 10.1016/j.ejpb.2023.10.003. Epub 2023 Oct 4. Eur J Pharm Biopharm. 2023. PMID: 37797679 Free PMC article.
-
Stabilizing HIV-1 envelope glycoprotein trimers to induce neutralizing antibodies.Retrovirology. 2018 Sep 12;15(1):63. doi: 10.1186/s12977-018-0445-y. Retrovirology. 2018. PMID: 30208933 Free PMC article. Review.
-
Structure-guided envelope trimer design in HIV-1 vaccine development: a narrative review.J Int AIDS Soc. 2021 Nov;24 Suppl 7(Suppl 7):e25797. doi: 10.1002/jia2.25797. J Int AIDS Soc. 2021. PMID: 34806305 Free PMC article. Review.
Cited by
-
Liposomal-Based Formulations: A Path from Basic Research to Temozolomide Delivery Inside Glioblastoma Tissue.Pharmaceutics. 2022 Jan 27;14(2):308. doi: 10.3390/pharmaceutics14020308. Pharmaceutics. 2022. PMID: 35214041 Free PMC article. Review.
-
Photothermal and radiotherapy with alginate-coated gold nanoparticles for breast cancer treatment.Sci Rep. 2024 Jun 10;14(1):13299. doi: 10.1038/s41598-024-60396-w. Sci Rep. 2024. PMID: 38858410 Free PMC article.
-
Design and Functional Characterization of HIV-1 Envelope Protein-Coupled T Helper Liposomes.Pharmaceutics. 2022 Jun 30;14(7):1385. doi: 10.3390/pharmaceutics14071385. Pharmaceutics. 2022. PMID: 35890282 Free PMC article.
-
Immunogenicity of Recombinant Lipid-Based Nanoparticle Vaccines: Danger Signal vs. Helping Hand.Pharmaceutics. 2023 Dec 23;16(1):24. doi: 10.3390/pharmaceutics16010024. Pharmaceutics. 2023. PMID: 38258035 Free PMC article. Review.
-
Site-Specific Antibody Assembly on Nanoparticles via a Versatile Coating Method for Improved Cell Targeting.Adv Sci (Weinh). 2023 Mar;10(9):e2206546. doi: 10.1002/advs.202206546. Epub 2023 Jan 25. Adv Sci (Weinh). 2023. PMID: 36698301 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

