Engineering and Preclinical Evaluation of Western Reserve Oncolytic Vaccinia Virus Expressing A167Y Mutant Herpes Simplex Virus Thymidine Kinase
- PMID: 33081279
- PMCID: PMC7650665
- DOI: 10.3390/biomedicines8100426
Engineering and Preclinical Evaluation of Western Reserve Oncolytic Vaccinia Virus Expressing A167Y Mutant Herpes Simplex Virus Thymidine Kinase
Abstract
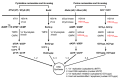
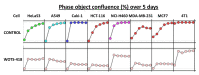
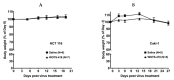
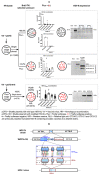
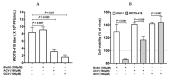
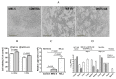
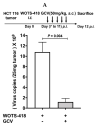
Viral replication of thymidine kinase deleted (tk-) vaccinia virus (VV) is attenuated in resting normal cells, enabling cancer selectivity, however, replication potency of VV-tk- appears to be diminished in cancer cells. Previously, we found that wild-type herpes simplex virus (HSV)-tk (HSV-tk) disappeared in most of the recombinant VV after multiple screenings, and only a few recombinant VV containing naturally mutated HSV-tk remained stable. In this study, VV-tk of western reserve (WR) VV was replaced by A167Y mutated HSV-tk (HSV-tk418m), to alter nucleoside selectivity from broad spectrum to purine exclusive selectivity. WOTS-418 remained stable after numerous passages. WOTS-418 replication was significantly attenuated in normal cells, but cytotoxicity was almost similar to that of wild type WR VV in cancer cells. WOTS-418 showed no lethality following a 5 × 108 PFU intranasal injection, contrasting WR VV, which showed 100% lethality at 1 × 105 PFU. Additionally, ganciclovir (GCV) but not BvdU inhibited WOTS-418 replication, confirming specificity to purine nucleoside analogs. The potency of WOTS-418 replication inhibition by GCV was > 10-fold higher than that of our previous truncated HSV-tk recombinant OTS-412. Overall, WOTS-418 demonstrated robust oncolytic efficacy and pharmacological safety which may delegate it as a candidate for future clinical use in OV therapy.
Keywords: A167Y; ganciclovir; herpes simplex virus; oncolytic viruses; thymidine kinase; western reserve vaccinia virus.
Conflict of interest statement
The authors declare no conflict of interest. T.H.H and M.C are the patent owner of the WOTS-418. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






Similar articles
-
Engineering and Characterization of Oncolytic Vaccinia Virus Expressing Truncated Herpes Simplex Virus Thymidine Kinase.Cancers (Basel). 2020 Jan 17;12(1):228. doi: 10.3390/cancers12010228. Cancers (Basel). 2020. PMID: 31963415 Free PMC article.
-
Selective abolishment of pyrimidine nucleoside kinase activity of herpes simplex virus type 1 thymidine kinase by mutation of alanine-167 to tyrosine.Mol Pharmacol. 2000 Dec;58(6):1326-32. doi: 10.1124/mol.58.6.1326. Mol Pharmacol. 2000. PMID: 11093770
-
The A167Y mutation converts the herpes simplex virus type 1 thymidine kinase into a guanosine analogue kinase.Biochemistry. 2002 May 21;41(20):6517-24. doi: 10.1021/bi0255930. Biochemistry. 2002. PMID: 12009916
-
The role of the E1B 55 kDa gene product in oncolytic adenoviral vectors expressing herpes simplex virus-tk: assessment of antitumor efficacy and toxicity.Cancer Res. 2000 Aug 1;60(15):4167-74. Cancer Res. 2000. PMID: 10945625
-
The Use of Oncolytic Viruses in the Treatment of Multiple Myeloma.Cancers (Basel). 2021 Nov 13;13(22):5687. doi: 10.3390/cancers13225687. Cancers (Basel). 2021. PMID: 34830842 Free PMC article. Review.
Cited by
-
Oncolytic Virus Immunotherapy: Showcasing Impressive Progress in Special Issue II.Biomedicines. 2021 Jun 10;9(6):663. doi: 10.3390/biomedicines9060663. Biomedicines. 2021. PMID: 34200560 Free PMC article.
-
Inhibition of MEK-ERK pathway enhances oncolytic vaccinia virus replication in doxorubicin-resistant ovarian cancer.Mol Ther Oncolytics. 2022 Apr 18;25:211-224. doi: 10.1016/j.omto.2022.04.006. eCollection 2022 Jun 16. Mol Ther Oncolytics. 2022. PMID: 35592390 Free PMC article.
-
Development of Allogeneic Stem Cell-Based Platform for Delivery and Potentiation of Oncolytic Virotherapy.Cancers (Basel). 2022 Dec 13;14(24):6136. doi: 10.3390/cancers14246136. Cancers (Basel). 2022. PMID: 36551636 Free PMC article.
-
Dose Considerations for Vaccinia Oncolytic Virus Based on Retrospective Reanalysis of Early and Late Clinical Trials.Vaccines (Basel). 2024 Sep 3;12(9):1010. doi: 10.3390/vaccines12091010. Vaccines (Basel). 2024. PMID: 39340040 Free PMC article.
-
"Non-Essential" Proteins of HSV-1 with Essential Roles In Vivo: A Comprehensive Review.Viruses. 2020 Dec 23;13(1):17. doi: 10.3390/v13010017. Viruses. 2020. PMID: 33374862 Free PMC article. Review.
References
-
- Park B.-H., Hwang T., Liu T.-C., Sze D.Y., Kim J.-S., Kwon H.-C., Oh S.Y., Han S.-Y., Yoon J.-H., Hong S.-H., et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: A phase I trial. Lancet Oncol. 2008;9:533–542. doi: 10.1016/S1470-2045(08)70107-4. - DOI - PubMed
-
- Hwang T.-H., Moon A., Burke J., Ribas A., Stephenson J., Breitbach C.J., Daneshmand M., De Silva N., Parato K., Diallo J.-S., et al. A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol. Ther. J. Am. Soc. Gene Ther. 2011;19:1913–1922. doi: 10.1038/mt.2011.132. - DOI - PMC - PubMed
-
- Downs-Canner S., Guo Z.S., Ravindranathan R., Breitbach C.J., O’Malley M.E., Jones H.L., Moon A., McCart J.A., Shuai Y., Zeh H.J., et al. Phase 1 Study of Intravenous Oncolytic Poxvirus (vvDD) in Patients with Advanced Solid Cancers. Mol. Ther. 2016;24:1492–1501. doi: 10.1038/mt.2016.101. - DOI - PMC - PubMed
-
- Kim S.G., Hwang T.H. 2597 Phase 2 trial of Pexa-Vec (pexastimogene devacirepvec; JX-594), an oncolytic and immunotherapeutic vaccinia virus, in patients with metastatic, refractory renal cell carcinoma (RCC) Eur. J. Cancer. 2015;51:S510. doi: 10.1016/S0959-8049(16)31415-0. - DOI
-
- Park S.H., Breitbach C.J., Lee J., Park J.O., Lim H.Y., Kang W.K., Moon A., Mun J.-H., Sommermann E.M., Maruri Avidal L., et al. Phase 1b Trial of Biweekly Intravenous Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus in Colorectal Cancer. Mol. Ther. 2015;23:1532–1540. doi: 10.1038/mt.2015.109. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

