Short-Chain Fatty Acids Modulate Metabolic Pathways and Membrane Lipids in Prevotella bryantii B14
- PMID: 33081314
- PMCID: PMC7709123
- DOI: 10.3390/proteomes8040028
Short-Chain Fatty Acids Modulate Metabolic Pathways and Membrane Lipids in Prevotella bryantii B14
Abstract
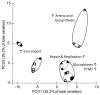
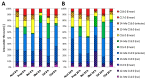
Short-chain fatty acids (SCFAs) are bacterial products that are known to be used as energy sources in eukaryotic hosts, whereas their role in the metabolism of intestinal microbes is rarely explored. In the present study, acetic, propionic, butyric, isobutyric, valeric, and isovaleric acid, respectively, were added to a newly defined medium containing Prevotella bryantii B14 cells. After 8 h and 24 h, optical density, pH and SCFA concentrations were measured. Long-chain fatty acid (LCFA) profiles of the bacterial cells were analyzed via gas chromatography-time of flight-mass spectrometry (GC-ToF MS) and proteins were quantified using a mass spectrometry-based, label-free approach. Cultures supplemented with single SCFAs revealed different growth behavior. Structural features of the respective SCFAs were identified in the LCFA profiles, which suggests incorporation into the bacterial membranes. The proteomes of cultures supplemented with acetic and valeric acid differed by an increased abundance of outer membrane proteins. The proteome of the isovaleric acid supplementation showed an increase of proteins in the amino acid metabolism. Our findings indicate a possible interaction between SCFAs, the lipid membrane composition, the abundance of outer membrane proteins, and a modulation of branched chain amino acid biosynthesis by isovaleric acid.
Keywords: Prevotella bryantii B14; branched-chain amino acids proteome; lipid membrane; long-chain fatty acids; outer membrane proteins; short-chain fatty acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Alterations in fecal short chain fatty acids (SCFAs) and branched short-chain fatty acids (BCFAs) in men with benign prostatic hyperplasia (BPH) and metabolic syndrome (MetS).Aging (Albany NY). 2021 Apr 13;13(8):10934-10954. doi: 10.18632/aging.202968. Epub 2021 Apr 13. Aging (Albany NY). 2021. PMID: 33847600 Free PMC article.
-
A novel LCMSMS method for quantitative measurement of short-chain fatty acids in human stool derivatized with 12C- and 13C-labelled aniline.J Pharm Biomed Anal. 2017 May 10;138:43-53. doi: 10.1016/j.jpba.2017.01.044. Epub 2017 Jan 28. J Pharm Biomed Anal. 2017. PMID: 28178633
-
Association of gut microbiota with sort-chain fatty acids and inflammatory cytokines in diabetic patients with cognitive impairment: A cross-sectional, non-controlled study.Front Nutr. 2022 Jul 22;9:930626. doi: 10.3389/fnut.2022.930626. eCollection 2022. Front Nutr. 2022. PMID: 35938126 Free PMC article.
-
Evaluation and comparison of short chain fatty acids composition in gut diseases.World J Gastroenterol. 2019 Sep 28;25(36):5543-5558. doi: 10.3748/wjg.v25.i36.5543. World J Gastroenterol. 2019. PMID: 31576099 Free PMC article.
-
Production of short-chain fatty acids (SCFAs) as chemicals or substrates for microbes to obtain biochemicals.Biotechnol Biofuels Bioprod. 2023 Jun 3;16(1):96. doi: 10.1186/s13068-023-02349-5. Biotechnol Biofuels Bioprod. 2023. PMID: 37270640 Free PMC article. Review.
Cited by
-
Central Carbon Metabolism, Sodium-Motive Electron Transfer, and Ammonium Formation by the Vaginal Pathogen Prevotella bivia.Int J Mol Sci. 2021 Nov 3;22(21):11925. doi: 10.3390/ijms222111925. Int J Mol Sci. 2021. PMID: 34769356 Free PMC article.
-
Effects of supplementation of sodium acetate on rumen fermentation and microbiota in postpartum dairy cows.Front Microbiol. 2022 Nov 21;13:1053503. doi: 10.3389/fmicb.2022.1053503. eCollection 2022. Front Microbiol. 2022. PMID: 36478854 Free PMC article.
-
A Sodium-Translocating Module Linking Succinate Production to Formation of Membrane Potential in Prevotella bryantii.Appl Environ Microbiol. 2021 Oct 14;87(21):e0121121. doi: 10.1128/AEM.01211-21. Epub 2021 Sep 1. Appl Environ Microbiol. 2021. PMID: 34469197 Free PMC article.
-
Interplay of human gastrointestinal microbiota metabolites: Short-chain fatty acids and their correlation with Parkinson's disease.Medicine (Baltimore). 2024 Apr 26;103(17):e37960. doi: 10.1097/MD.0000000000037960. Medicine (Baltimore). 2024. PMID: 38669388 Free PMC article. Review.
-
Metagenomic analysis reveals rumen microbiome enrichment and functional genes adjustment in carbohydrate metabolism induced by different sorting behavior in mid-lactation dairy cows.Anim Microbiome. 2025 Jul 28;7(1):82. doi: 10.1186/s42523-025-00439-3. Anim Microbiome. 2025. PMID: 40722120 Free PMC article.
References
-
- Duncan S.H., Hold G.L., Harmsen H.J., Stewart C.S., Flint H.J. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2002;52:2141–2146. - PubMed
-
- Torrungruang K., Jitpakdeebordin S., Charatkulangkun O., Gleebbua Y. Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Treponema denticola/Prevotella intermedia co-infection are associated with severe periodontitis in a thai population. PLoS ONE. 2015;10:e0136646. doi: 10.1371/journal.pone.0136646. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

