Copper Toxicity Links to Pathogenesis of Alzheimer's Disease and Therapeutics Approaches
- PMID: 33081348
- PMCID: PMC7589751
- DOI: 10.3390/ijms21207660
Copper Toxicity Links to Pathogenesis of Alzheimer's Disease and Therapeutics Approaches
Abstract
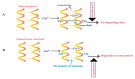
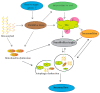
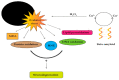
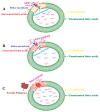
Alzheimer's disease (AD) is an irreversible, age-related progressive neurological disorder, and the most common type of dementia in aged people. Neuropathological lesions of AD are neurofibrillary tangles (NFTs), and senile plaques comprise the accumulated amyloid-beta (Aβ), loaded with metal ions including Cu, Fe, or Zn. Some reports have identified metal dyshomeostasis as a neurotoxic factor of AD, among which Cu ions seem to be a central cationic metal in the formation of plaque and soluble oligomers, and have an essential role in the AD pathology. Cu-Aβ complex catalyzes the generation of reactive oxygen species (ROS) and results in oxidative damage. Several studies have indicated that oxidative stress plays a crucial role in the pathogenesis of AD. The connection of copper levels in AD is still ambiguous, as some researches indicate a Cu deficiency, while others show its higher content in AD, and therefore there is a need to increase and decrease its levels in animal models, respectively, to study which one is the cause. For more than twenty years, many in vitro studies have been devoted to identifying metals' roles in Aβ accumulation, oxidative damage, and neurotoxicity. Towards the end, a short review of the modern therapeutic approach in chelation therapy, with the main focus on Cu ions, is discussed. Despite the lack of strong proofs of clinical advantage so far, the conjecture that using a therapeutic metal chelator is an effective strategy for AD remains popular. However, some recent reports of genetic-regulating copper transporters in AD models have shed light on treating this refractory disease. This review aims to succinctly present a better understanding of Cu ions' current status in several AD features, and some conflicting reports are present herein.
Keywords: Alzheimer’s disease; amyloid plaques; copper; neurodegeneration; oxidative damages; protein modification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Testai F.D., Gorelick P.B. Stroke Revisited: Vascular Cognitive Impairment. Springer; Berlin/Heidelberg, Germany: 2020. Definition and Concept of Vascular Cognitive Impairment; pp. 1–14.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

