Interactions of Gold and Silver Nanoparticles with Bacterial Biofilms: Molecular Interactions behind Inhibition and Resistance
- PMID: 33081366
- PMCID: PMC7589962
- DOI: 10.3390/ijms21207658
Interactions of Gold and Silver Nanoparticles with Bacterial Biofilms: Molecular Interactions behind Inhibition and Resistance
Abstract
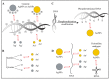
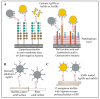
Many bacteria have the capability to form a three-dimensional, strongly adherent network called 'biofilm'. Biofilms provide adherence, resourcing nutrients and offer protection to bacterial cells. They are involved in pathogenesis, disease progression and resistance to almost all classical antibiotics. The need for new antimicrobial therapies has led to exploring applications of gold and silver nanoparticles against bacterial biofilms. These nanoparticles and their respective ions exert antimicrobial action by damaging the biofilm structure, biofilm components and hampering bacterial metabolism via various mechanisms. While exerting the antimicrobial activity, these nanoparticles approach the biofilm, penetrate it, migrate internally and interact with key components of biofilm such as polysaccharides, proteins, nucleic acids and lipids via electrostatic, hydrophobic, hydrogen-bonding, Van der Waals and ionic interactions. Few bacterial biofilms also show resistance to these nanoparticles through similar interactions. The nature of these interactions and overall antimicrobial effect depend on the physicochemical properties of biofilm and nanoparticles. Hence, study of these interactions and participating molecular players is of prime importance, with which one can modulate properties of nanoparticles to get maximal antibacterial effects against a wide spectrum of bacterial pathogens. This article provides a comprehensive review of research specifically directed to understand the molecular interactions of gold and silver nanoparticles with various bacterial biofilms.
Keywords: antimicrobials; biofilm; biofilm inhibition; gold nanoparticles (AuNPs); molecular interactions; silver nanoparticles (AgNPs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications.Artif Cells Nanomed Biotechnol. 2018 Sep;46(6):1163-1170. doi: 10.1080/21691401.2017.1362417. Epub 2017 Aug 8. Artif Cells Nanomed Biotechnol. 2018. PMID: 28784039
-
The effect of gold and silver nanoparticles, chitosan and their combinations on bacterial biofilms of food-borne pathogens.Biofouling. 2020 Feb;36(2):222-233. doi: 10.1080/08927014.2020.1751132. Epub 2020 Apr 22. Biofouling. 2020. PMID: 32316774
-
The Polymeric Matrix Composition of Vibrio cholerae Biofilms Modulate Resistance to Silver Nanoparticles Prepared by Hydrothermal Synthesis.ACS Appl Mater Interfaces. 2021 Aug 4;13(30):35356-35364. doi: 10.1021/acsami.1c07455. Epub 2021 Jul 21. ACS Appl Mater Interfaces. 2021. PMID: 34286588
-
Antibacterial and Anti-Biofilm Biosynthesised Silver and Gold Nanoparticles for Medical Applications: Mechanism of Action, Toxicity and Current Status.Curr Drug Deliv. 2020;17(2):88-100. doi: 10.2174/1567201817666191227094334. Curr Drug Deliv. 2020. PMID: 31880259 Review.
-
Silver nanoparticles as an alternative strategy against bacterial biofilms.Acta Biochim Pol. 2013;60(4):523-30. Acta Biochim Pol. 2013. PMID: 24432308 Review.
Cited by
-
Green synthesis and antibacterial applications of gold and silver nanoparticles from Ligustrum vulgare berries.Sci Rep. 2022 May 12;12(1):7902. doi: 10.1038/s41598-022-11811-7. Sci Rep. 2022. PMID: 35551489 Free PMC article.
-
Current Knowledge on the Oxidative-Stress-Mediated Antimicrobial Properties of Metal-Based Nanoparticles.Microorganisms. 2022 Feb 14;10(2):437. doi: 10.3390/microorganisms10020437. Microorganisms. 2022. PMID: 35208891 Free PMC article. Review.
-
The effect of different intracanal medicaments on the dislodgement resistance of mineral trioxide aggregate.BMC Oral Health. 2022 May 25;22(1):207. doi: 10.1186/s12903-022-02213-2. BMC Oral Health. 2022. PMID: 35614409 Free PMC article.
-
Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins.Open Life Sci. 2025 Feb 25;20(1):20221045. doi: 10.1515/biol-2022-1045. eCollection 2025. Open Life Sci. 2025. PMID: 40026363 Free PMC article.
-
CLINICALLY RELEVANT METALLIC NANOPARTICLES IN TUBERCULOSIS DIAGNOSIS AND THERAPY.Adv Ther (Weinh). 2025 Apr;8(4):2400189. doi: 10.1002/adtp.202400189. Epub 2024 Aug 20. Adv Ther (Weinh). 2025. PMID: 40631045 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

