Binding Mode Exploration of B1 Receptor Antagonists' by the Use of Molecular Dynamics and Docking Simulation-How Different Target Engagement Can Determine Different Biological Effects
- PMID: 33081372
- PMCID: PMC7590058
- DOI: 10.3390/ijms21207677
Binding Mode Exploration of B1 Receptor Antagonists' by the Use of Molecular Dynamics and Docking Simulation-How Different Target Engagement Can Determine Different Biological Effects
Abstract
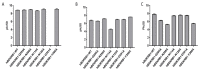
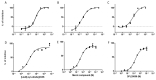
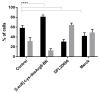
The kinin B1 receptor plays a critical role in the chronic phase of pain and inflammation. The development of B1 antagonists peaked in recent years but almost all promising molecules failed in clinical trials. Little is known about these molecules' mechanisms of action and additional information will be necessary to exploit the potential of the B1 receptor. With the aim of contributing to the available knowledge of the pharmacology of B1 receptors, we designed and characterized a novel class of allosteric non-peptidic inhibitors with peculiar binding characteristics. Here, we report the binding mode analysis and pharmacological characterization of a new allosteric B1 antagonist, DFL20656. We analyzed the binding of DFL20656 by single point mutagenesis and radioligand binding assays and we further characterized its pharmacology in terms of IC50, B1 receptor internalization and in vivo activity in comparison with different known B1 antagonists. We highlighted how different binding modes of DFL20656 and a Merck compound (compound 14) within the same molecular pocket can affect the biological and pharmacological properties of B1 inhibitors. DFL20656, by its peculiar binding mode, involving tight interactions with N114, efficiently induced B1 receptor internalization and evoked a long-lasting effect in an in vivo model of neuropathic pain. The pharmacological characterization of different B1 antagonists highlighted the effects of their binding modes on activity, receptor occupancy and internalization. Our results suggest that part of the failure of most B1 inhibitors could be ascribed to a lack of knowledge about target function and engagement.
Keywords: allosteric inhibitors; biased signaling; bradykinin 1; neuropathic pain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
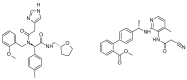


References
-
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharm. Rev. 1980;32:1–46. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

