Facts and Challenges in Immunotherapy for T-Cell Acute Lymphoblastic Leukemia
- PMID: 33081391
- PMCID: PMC7589289
- DOI: 10.3390/ijms21207685
Facts and Challenges in Immunotherapy for T-Cell Acute Lymphoblastic Leukemia
Abstract
T-cell acute lymphoblastic leukemia (T-ALL), a T-cell malignant disease that mainly affects children, is still a medical challenge, especially for refractory patients for whom therapeutic options are scarce. Recent advances in immunotherapy for B-cell malignancies based on increasingly efficacious monoclonal antibodies (mAbs) and chimeric antigen receptors (CARs) have been encouraging for non-responding or relapsing patients suffering from other aggressive cancers like T-ALL. However, secondary life-threatening T-cell immunodeficiency due to shared expression of targeted antigens by healthy and malignant T cells is a main drawback of mAb-or CAR-based immunotherapies for T-ALL and other T-cell malignancies. This review provides a comprehensive update on the different immunotherapeutic strategies that are being currently applied to T-ALL. We highlight recent progress on the identification of new potential targets showing promising preclinical results and discuss current challenges and opportunities for developing novel safe and efficacious immunotherapies for T-ALL.
Keywords: T-cell acute lymphoblastic leukemia; chimeric antigen receptor; immunotherapy; leukemia-initiating cells; monoclonal antibodies; relapse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


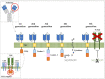
References
-
- Borella L., Sen L. T cell surface markers on lymphoblasts from acute lymphocytic leukemia. J. Immunol. 1973;111:1257–1260. - PubMed
-
- Goldberg J.M., Silverman L.B., Levy D.E., Dalton V.K., Gelber R.D., Lehmann L., Cohen H.J., Sallan S.E., Asselin B.L. Childhood T-cell acute lymphoblastic leukemia: The Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium experience. J. Clin. Oncol. 2003;21:3616–3622. doi: 10.1200/JCO.2003.10.116. - DOI - PubMed
-
- Bene M.C., Castoldi G., Knapp W., Ludwig W.D., Matutes E., Orfao A., Van’t Veer M.B. Proposals for the immunological classification of acute leukemias. Leukemia. 1995;9:1783–1786. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

