The Protective Role of Bioactive Quinones in Stress-induced Senescence Phenotype of Endothelial Cells Exposed to Cigarette Smoke Extract
- PMID: 33081423
- PMCID: PMC7602940
- DOI: 10.3390/antiox9101008
The Protective Role of Bioactive Quinones in Stress-induced Senescence Phenotype of Endothelial Cells Exposed to Cigarette Smoke Extract
Abstract
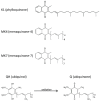
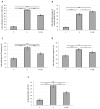
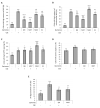
Endothelial dysfunction represents the initial stage in atherosclerotic lesion development which occurs physiologically during aging, but external factors like diet, sedentary lifestyle, smoking accelerate it. Since cigarette smoking promotes oxidative stress and cell damage, we developed an in vitro model of endothelial dysfunction using vascular cells exposed to chemicals present in cigarette smoke, to help elucidate the protective effects of anti-inflammatory and antioxidant agents, such as ubiquinol and vitamin K, that play a fundamental role in vascular health. Treatment of both young and senescent Human Umbilical Vein Endothelial Cells (HUVECs) for 24 h with cigarette smoke extract (CSE) decreased cellular viability, induced apoptosis via reactive oxygen species (ROS) imbalance and mitochondrial dysfunction and promoted an inflammatory response. Moreover, the senescence marker SA-β-galactosidase was observed in both young CSE-exposed and in senescent HUVECs suggesting that CSE exposure accelerates aging in endothelial cells. Supplementation with 10 µM ubiquinol and menaquinone-7 (MK7) counteracted oxidative stress and inflammation, resulting in improved viability, decreased apoptosis and reduced SA-β-galactosidase, but were ineffective against CSE-induced mitochondrial permeability transition pore opening. Other K vitamins tested like menaquinone-4 (MK4) and menaquinone-1 (K1) were less protective. In conclusion, CSE exposure was able to promote a stress-induced senescent phenotype in young endothelial cells likely contributing to endothelial dysfunction in vivo. Furthermore, the molecular changes encountered could be offset by ubiquinol and menaquinone-7 supplementation, the latter resulting the most bioactive K vitamin in counteracting CSE-induced damage.
Keywords: aging; cigarette smoke; endothelial dysfunction; menaquinone; mitochondrial dysfunction; oxidative stress; ubiquinol; vitamin K.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Rodgman A., Perfetti T.A. The Chemical Components of Tobacco and Tobacco Smoke. 2nd ed. CRC Press; Boca Raton, FL, USA: 2013. pp. 1221–1297.
-
- Farhat N., Thorin-Trescases N., Voghel G., Villeneuve L., Mamarbachi M., Perrault L.P., Carrier M., Thorin E. Stress-induced senescence predominates in endothelial cells isolated from atherosclerotic chronic smokers. Can. J. Physiol. Pharmacol. 2008;86:761–769. doi: 10.1139/Y08-082. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

