Synthesis, Structural Characterization, and DFT Investigations of [MxM'5- xFe4(CO)16]3- (M, M' = Cu, Ag, Au; M ≠ M') 2-D Molecular Alloy Clusters
- PMID: 33081462
- PMCID: PMC8015236
- DOI: 10.1021/acs.inorgchem.0c02443
Synthesis, Structural Characterization, and DFT Investigations of [MxM'5- xFe4(CO)16]3- (M, M' = Cu, Ag, Au; M ≠ M') 2-D Molecular Alloy Clusters
Abstract
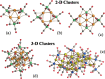
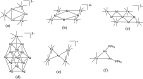
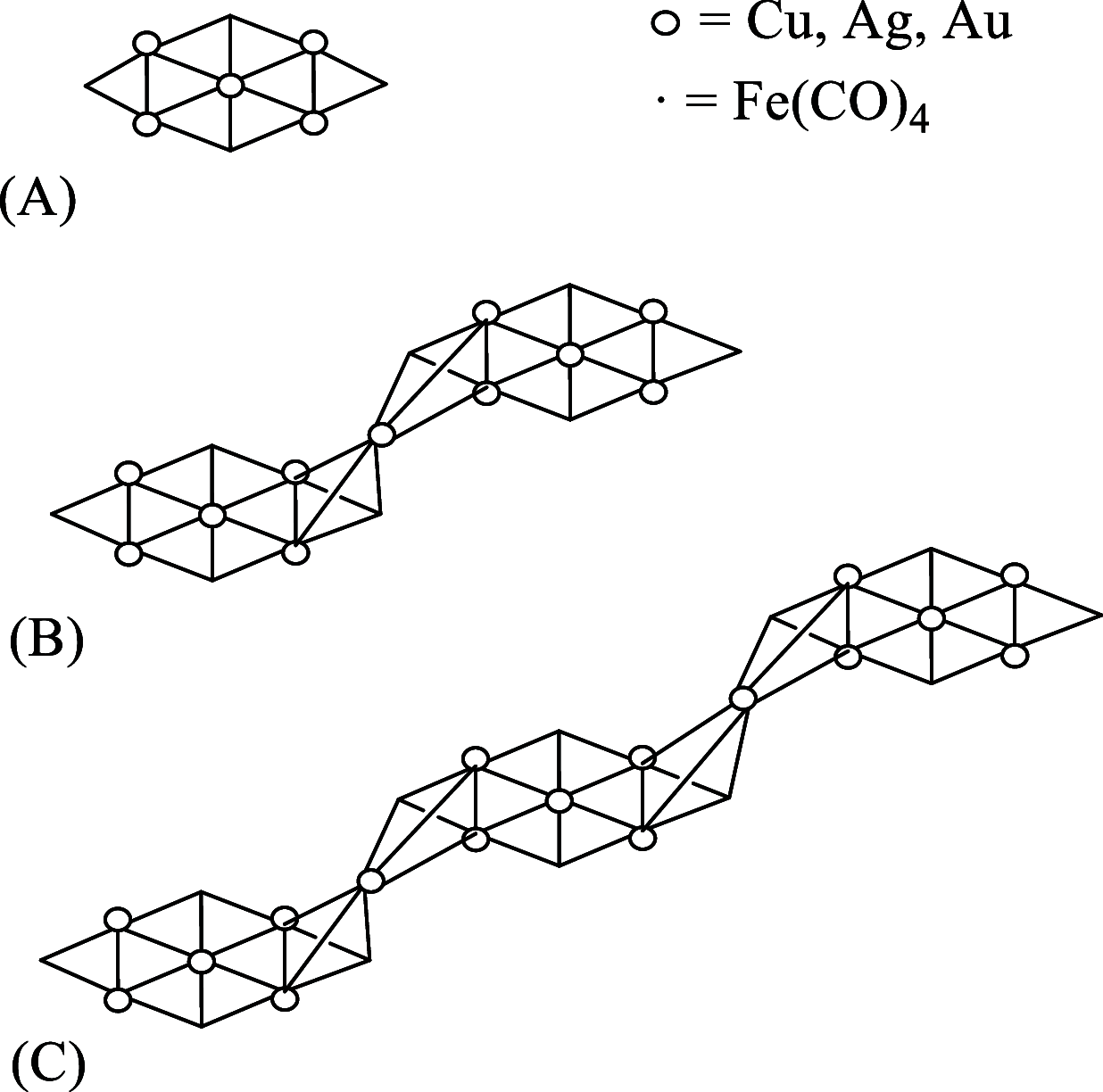
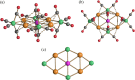
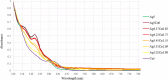
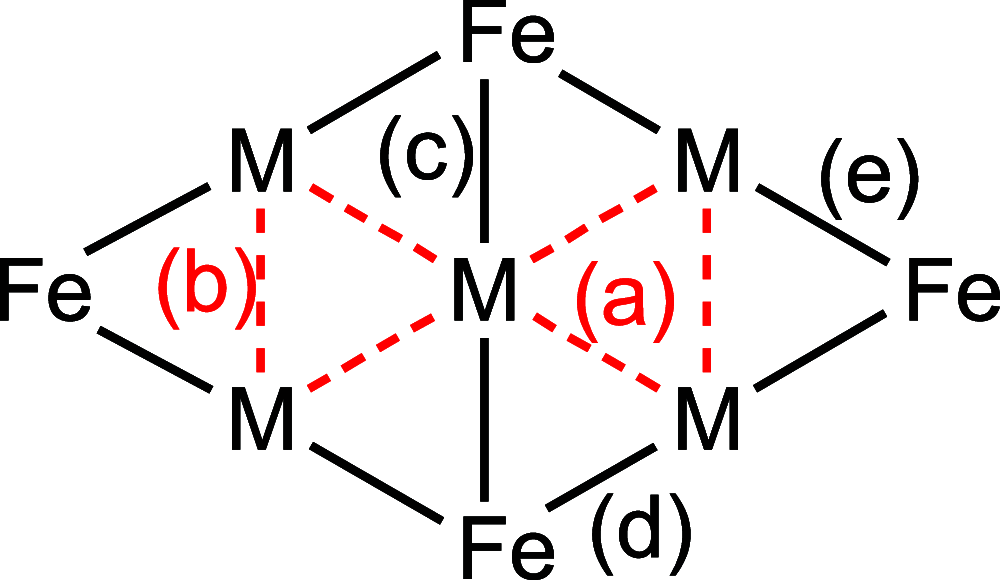
Miscellaneous 2-D molecular alloy clusters of the type [MxM'5-xFe4(CO)16]3- (M, M' = Cu, Ag, Au; M ≠ M') have been prepared through the reactions of [Cu3Fe3(CO)12]3-, [Ag4Fe4(CO)16]4- or [M5Fe4(CO)16]3- (M = Cu, Ag) with M'(I) salts (M' = Cu, Ag, Au). Their formation involves a combination of oxidation, condensation, and substitution reactions. The total structures of several [MxM'5-xFe4(CO)16]3- clusters with different compositions have been determined by means of single crystal X-ray diffraction (SC-XRD) and their nature in solution elucidated by electron spray ionization mass spectrometry (ESI-MS) and IR and UV-visible spectroscopy. Substitutional and compositional disorder is present in the solid state structures, and ESI-MS analyses point out that mixtures of isostructural clusters differing by a few M/M' coinage metals are present. SC-XRD studies indicate some site preferences of the coinage metals within the metal cores of these clusters, with Au preferentially in corner sites and Cu in the central site. DFT studies give theoretical support to the experimental structural evidence. The site preference is mainly dictated by the strength of the Fe-M bonds that decreases in the order Fe-Au > Fe-Ag > Fe-Cu, and the preferred structure is the one that maximizes the number of stronger Fe-M interactions. Overall, the molecular nature of these clusters allows their structures to be fully revealed with atomic precision, resulting in the elucidation of the bonding parameters that determine the distribution of the different metals within their metal cores.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


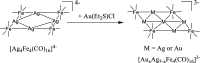



Similar articles
-
Electronic structures and nonlinear optical properties of trinuclear transition metal clusters M-(mu-S)-M' (M = Mo, W; M' = Cu, Ag, Au).Inorg Chem. 2003 Jan 27;42(2):532-40. doi: 10.1021/ic025649e. Inorg Chem. 2003. PMID: 12693236
-
Metallophilic interactions: observations of the shortest metallophilicinteractions between closed shell (d10d10, d10d8, d8d8) metal ions [MM' M = Hg(ii) and Pd(ii) and M' = Cu(i), Ag(i), Au(i), and Pd(ii)].Dalton Trans. 2020 Jul 14;49(26):9099-9117. doi: 10.1039/d0dt01008a. Epub 2020 Jun 23. Dalton Trans. 2020. PMID: 32573621
-
Mixed copper, silver, and gold cyanides, (M(x)M'(1-x))CN: tailoring chain structures to influence physical properties.J Am Chem Soc. 2012 Oct 3;134(39):16387-400. doi: 10.1021/ja307087d. Epub 2012 Sep 20. J Am Chem Soc. 2012. PMID: 22954066
-
Recent progress in dichalcophosphate coinage metal clusters and superatoms.Chem Commun (Camb). 2023 Jun 8;59(47):7182-7195. doi: 10.1039/d3cc01215h. Chem Commun (Camb). 2023. PMID: 37184074 Review.
-
Lessons from recent theoretical treatments of Al-M bonds (M = Fe, Cu, Ag, Au) that capture CO2.Dalton Trans. 2024 Aug 20;53(33):13709-13715. doi: 10.1039/d4dt02018a. Dalton Trans. 2024. PMID: 39106074 Review.
Cited by
-
Heterometallic Ni-Pt Chini-Type Carbonyl Clusters: An Example of Molecular Random Alloy Clusters.Inorg Chem. 2021 Jun 21;60(12):8811-8825. doi: 10.1021/acs.inorgchem.1c00752. Epub 2021 Jun 3. Inorg Chem. 2021. PMID: 34082535 Free PMC article.
-
Polymerization Isomerism in Co-M (M = Cu, Ag, Au) Carbonyl Clusters: Synthesis, Structures and Computational Investigation.Molecules. 2021 Mar 11;26(6):1529. doi: 10.3390/molecules26061529. Molecules. 2021. PMID: 33799629 Free PMC article.
-
2-D Molecular Alloy Ru-M (M = Cu, Ag, and Au) Carbonyl Clusters: Synthesis, Molecular Structure, Catalysis, and Computational Studies.Inorg Chem. 2022 Sep 19;61(37):14726-14741. doi: 10.1021/acs.inorgchem.2c02099. Epub 2022 Sep 7. Inorg Chem. 2022. PMID: 36069711 Free PMC article.
-
Atomically Precise Ni-Pd Alloy Carbonyl Nanoclusters: Synthesis, Total Structure, Electrochemistry, Spectroelectrochemistry, and Electrochemical Impedance Spectroscopy.Inorg Chem. 2021 Nov 1;60(21):16713-16725. doi: 10.1021/acs.inorgchem.1c02582. Epub 2021 Oct 21. Inorg Chem. 2021. PMID: 34672566 Free PMC article.
References
-
- Sun W.; Jin S.; Du W.; Kang X.; Chen A.; Wang S.; Sheng H.; Zhu M. Total Structure Determination of the Pt1Ag9[P(Ph-F)3]7Cl3 Nanocluster. Eur. J. Inorg. Chem. 2020, 2020, 590–594. 10.1002/ejic.201901271. - DOI
-
- Higaki T.; Liu C.; Morris D. J.; He G.; Luo T.-Y.; Sfeir M.; Zhang P.; Rosi N. L.; Jin R. Au130-xAgx Nanoclusters with Non-Metallicity: A Drum of Silver-Rich Sites Enclosed in a Marks-Decahedral Cage of Gold-Rich Sites. Angew. Chem., Int. Ed. 2019, 58, 18798–18802. 10.1002/anie.201908694. - DOI - PubMed
LinkOut - more resources
Full Text Sources

