Effect of Empagliflozin on the Clinical Stability of Patients With Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial
- PMID: 33081531
- PMCID: PMC7834905
- DOI: 10.1161/CIRCULATIONAHA.120.051783
Effect of Empagliflozin on the Clinical Stability of Patients With Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial
Erratum in
-
Correction to: Effect of Empagliflozin on the Clinical Stability of Patients With Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial.Circulation. 2021 Jan 26;143(4):e30. doi: 10.1161/CIR.0000000000000954. Epub 2021 Jan 25. Circulation. 2021. PMID: 33493036 Free PMC article. No abstract available.
Abstract
Background: Empagliflozin reduces the risk of cardiovascular death or hospitalization for heart failure in patients with heart failure and a reduced ejection fraction, with or without diabetes, but additional data are needed about the effect of the drug on inpatient and outpatient events that reflect worsening heart failure.
Methods: We randomly assigned 3730 patients with class II to IV heart failure with an ejection fraction of ≤40% to double-blind treatment with placebo or empagliflozin (10 mg once daily), in addition to recommended treatments for heart failure, for a median of 16 months. We prospectively collected information on inpatient and outpatient events reflecting worsening heart failure and prespecified their analysis in individual and composite end points.
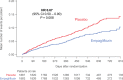
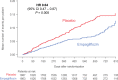
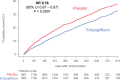
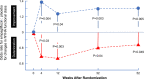
Results: Empagliflozin reduced the combined risk of death, hospitalization for heart failure or an emergent/urgent heart failure visit requiring intravenous treatment (415 versus 519 patients; empagliflozin versus placebo, respectively; hazard ratio [HR], 0.76; 95% CI, 0.67-0.87; P<0.0001). This benefit reached statistical significance at 12 days after randomization. Empagliflozin reduced the total number of heart failure hospitalizations that required intensive care (HR, 0.67; 95% CI, 0.50-0.90; P=0.008) and that required a vasopressor or positive inotropic drug or mechanical or surgical intervention (HR, 0.64; 95% CI, 0.47-0.87; P=0.005). As compared with placebo, fewer patients in the empagliflozin group reported intensification of diuretics (297 versus 414 [HR, 0.67; 95% CI, 0.56-0.78; P<0.0001]). Additionally, patients assigned to empagliflozin were 20% to 40% more likely to experience an improvement in New York Heart Association functional class and were 20% to 40% less likely to experience worsening of New York Heart Association functional class, with statistically significant effects that were apparent 28 days after randomization and maintained during long-term follow-up. The risk of any inpatient or outpatient worsening heart failure event in the placebo group was high (48.1 per 100 patient-years of follow-up), and it was reduced by empagliflozin (HR, 0.70; 95% CI, 0.63-0.78; P<0.0001).
Conclusions: In patients with heart failure and a reduced ejection fraction, empagliflozin reduced the risk and total number of inpatient and outpatient worsening heart failure events, with benefits seen early after initiation of treatment and sustained for the duration of double-blind therapy. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03057977.
Keywords: empagliflozin; heart failure; sodium-glucose transporter 2 inhibitors.
Conflict of interest statement
Dr Packer reports consulting fees from Boehringer Ingelheim and Akcea received during the conduct of the study; and consulting fees from Abbvie, Akcea, Amarin, AstraZeneca, Amgen, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Johnson & Johnson, Lilly, Novartis, Pfizer, Relypsa, Sanofi, Synthetic Biologics, Theravance, and NovoNordisk, all outside of the submitted work. Dr Anker reports grants and/or personal fees from Abbott Vascular, AstraZeneca, Bayer, Brahms, Boehringer Ingelheim, Cardiac Dimensions, Novartis, Occlutech, Respircardia, Servier, and Vifor Pharma; and personal fees from Boehringer Ingelheim received during the conduct of the study. Dr Butler reports research support from the National Institutes of Health, Patient Centered Outcomes Research and the European Union, and consulting fees from Abbott, Adrenomed, Amgen, Array, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CVRx, G3 Pharmaceutical, Innolife, Janssen, LinaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, V-Wave Limited, and Vifor. Dr Fillipatos reports consulting fees from Boehringer Ingelheim during the study and consulting/lecture fees from Novartis, Vifor, Servier, Medtronic and Merck. Dr Ferreira is a consultant for Boehringer Ingelheim. Dr Pocock is a consultant for Boehringer Ingelheim, and reports personal fees received from Boehringer Ingelheim during the conduct of the study. Dr Carson reports consulting fees from Boehringer Ingelheim and IQVIA related to work on the Clinical Events Committee during the conduct of the study. Dr Anand reports personal/consulting fees from ARCA, Amgen, Boston Scientific Corporation, Novartis, LivaNova, and Zensun. Dr Doehner reports personal fees from Aimediq, Bayer, Boehringer Ingelheim, Medtronic, Pfizer, Sanofi-Aventis, Sphingotec, and Vifor Pharma; and research support from the European Union (Horizon2020), German Ministry of Education and Research, German Center for Cardiovascular Research, Vifor Pharma, and ZS Pharma. Dr Haass reports consulting fees from Boehringer Ingelheim related to work on the Clinical Events Committee during the conduct of the study. Dr Komajda reports consulting fees from Boehringer Ingelheim related to work on the Clinical Events Committee during the conduct of the study; and personal fees from Novartis, Servier, Amgen, Sanofi, Bayer, AstraZeneca, Lilly, and Torrent. Dr Miller reports consulting fees from Abbott, Boehringer Ingelheim, Respicardia, CVRx, Pfizer, and Abbvie. Dr Pehrson reports consulting fees and/or lecture fees from Boehringer Ingelheim, Glaxo Smith Kline, Celgene, Bristol Myers Squibb, Bayer, Johnson & Johnson. Dr Teerlink reports grants and/or consulting fees from Abbott, Amgen, Astra-Zeneca, Bayer, Boehringer-Ingelheim, Cytokinetics, Daxor, EBR Systems, LivaNova, Medtronic, Merck, Novartis, Relypsa, Servier, Windtree Therapeutics, and ZS Pharma. Drs Brueckmann and Jamal, C. Zeller, and S. Schnaidt are employees of Boehringer Ingelheim. Dr Zannad has received steering committee or advisory board fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cardior, CVRx, Janssen, Livanova, Merck, Mundipharma, Novartis, Novo Nordisk, and Vifor Fresenius; and personal fees from Boehringer Ingelheim during the conduct of the study.
Figures
Comment in
-
Empagliflozin improves clinical outcomes for HFpEF in EMPEROR-Preserved.Nat Rev Cardiol. 2021 Nov;18(11):737. doi: 10.1038/s41569-021-00627-z. Nat Rev Cardiol. 2021. PMID: 34526679 No abstract available.
References
-
- Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME® trial investigators. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016; 37:1526–1534. doi: 10.1093/eurheartj/ehv728 - PMC - PubMed
-
- Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL, Leiter LA, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019; 139:2528–2536. doi: 10.1161/CIRCULATIONAHA.119.040130 - PubMed
-
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. ; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380:2295–2306. doi: 10.1056/NEJMoa1811744 - PubMed
-
- Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, et al. ; VERTIS CV Investigators. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020; 383:1425–1435. doi: 10.1056/NEJMoa2004967 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

