The biophysical basis of thermal tolerance in fish eggs
- PMID: 33081621
- PMCID: PMC7661308
- DOI: 10.1098/rspb.2020.1550
The biophysical basis of thermal tolerance in fish eggs
Abstract
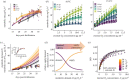
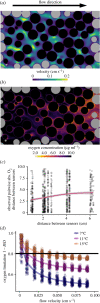
A warming climate poses a fundamental problem for embryos that develop within eggs because their demand for oxygen (O2) increases much more rapidly with temperature than their capacity for supply, which is constrained by diffusion across the egg surface. Thus, as temperatures rise, eggs may experience O2 limitation due to an imbalance between O2 supply and demand. Here, we formulate a mathematical model of O2 limitation and experimentally test whether this mechanism underlies the upper thermal tolerance in large aquatic eggs. Using Chinook salmon (Oncorhynchus tshawytscha) as a model system, we show that the thermal tolerance of eggs varies systematically with features of the organism and environment. Importantly, this variation can be precisely predicted by the degree to which these features shift the balance between O2 supply and demand. Equipped with this mechanistic understanding, we predict and experimentally confirm that the thermal tolerance of these embryos in their natural habitat is substantially lower than expected from laboratory experiments performed under normoxia. More broadly, our biophysical model of O2 limitation provides a mechanistic explanation for the elevated thermal sensitivity of fish embryos relative to other life stages, global patterns in egg size and the extreme fecundity of large teleosts.
Keywords: egg; embryo; metabolic rate; oxygen limitation; temperature; thermal tolerance.
Conflict of interest statement
We declare we have no competing interests.
Figures


References
-
- McCullough DA.1999. A review and synthesis of effects of alterations to the water temperature regime on freshwater life stages of salmonids, with special reference to Chinook salmon. Seattle, WA: US Environmental Protection Agency.
