The structure and function of deubiquitinases: lessons from budding yeast
- PMID: 33081638
- PMCID: PMC7653365
- DOI: 10.1098/rsob.200279
The structure and function of deubiquitinases: lessons from budding yeast
Abstract
Protein ubiquitination is a key post-translational modification that regulates diverse cellular processes in eukaryotic cells. The specificity of ubiquitin (Ub) signalling for different bioprocesses and pathways is dictated by the large variety of mono-ubiquitination and polyubiquitination events, including many possible chain architectures. Deubiquitinases (DUBs) reverse or edit Ub signals with high sophistication and specificity, forming an integral arm of the Ub signalling machinery, thus impinging on fundamental cellular processes including DNA damage repair, gene expression, protein quality control and organellar integrity. In this review, we discuss the many layers of DUB function and regulation, with a focus on insights gained from budding yeast. Our review provides a framework to understand key aspects of DUB biology.
Keywords: deubiquitinases; protein degradation; ubiquitin signalling.
Conflict of interest statement
We declare we have no competing interests.
Figures
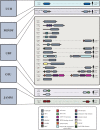

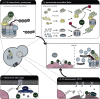
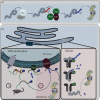

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
