Urinary nitrate concentration as a marker for kidney transplant rejection
- PMID: 33081704
- PMCID: PMC7576839
- DOI: 10.1186/s12882-020-02096-x
Urinary nitrate concentration as a marker for kidney transplant rejection
Abstract
Background: Early identification and treatment of kidney transplant rejection episodes is vital to limit loss of function and prolong the life of the transplanted kidney and recipient. Current practice depends on detecting a creatinine rise. A biomarker to diagnose transplant rejection at an earlier time point than current practice, or to inform earlier decision making to biopsy, could be transformative. It has previously been shown that urinary nitrate concentration is elevated in renal transplant rejection. Nitrate is a nitric oxide (NO) oxidation product. Transplant rejection upregulates NO synthesis via inducible nitric oxide synthase leading to elevations in urinary nitrate concentration. We have recently validated a urinary nitrate concentration assay which could provide results in a clinically relevant timeframe. Our aim was to determine whether urinary nitrate concentration is a useful tool to predict renal transplant rejection in the context of contemporary clinical practice.
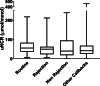
Methods: We conducted a prospective observational study, recruiting renal transplant participants over an 18-month period. We made no alterations to the patients' clinical care including medications, immunosuppression, diet and frequency of visits. We collected urine samples from every clinical attendance. We assessed the urinary nitrate to creatinine ratio (uNCR) between patient groups: routine attendances, biopsy proven rejection, biopsy proven no rejection and other call backs. uNCR was examined over time for those with biopsy proven transplant rejection. These four groups were compared using an ANOVA test.
Results: A total of 2656 samples were collected. uNCR during biopsy proven rejection, n = 15 (median 49 μmol/mmol, IQR 23-61) was not significantly different from that of routine samples, n = 164 (median 55 μmol/mmol, IQR 37-82) (p = 0.55), or biopsy proven no rejection, n = 12 (median 39 μmol/mmol, IQR 21-89) (P = 0.77). Overall uNCR was highly variable with no diagnostic threshold for kidney transplant rejection. Furthermore, within-patient uNCR was highly variable over time, and thus it was not possible to produce individualised patient thresholds to identify rejection. The total taking Tacrolimus was 204 patients, with no statistical difference between the uNCR of all those on Tacrolimus, against those not, p = 0.18.
Conclusion: The urinary nitrate to creatinine ratio is not a useful biomarker for renal transplant rejection.
Keywords: Biomarker; Kidney; Nitrate; Rejection; Transplant.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
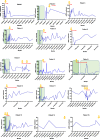
 1st month post transplant
1st month post transplant  Biopsy proven rejection.
Biopsy proven rejection.  Months 2-3 post tranmsplant
Months 2-3 post tranmsplant  1 month post confirmed rejection
1 month post confirmed rejectionReferences
-
- Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, Nankivell BJ, Halloran PF, et al. The Banff 2017 kidney meeting report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next generation clinical trials. Am J Transplant. 2018;18(2):293–307. doi: 10.1111/ajt.14625. - DOI - PMC - PubMed
-
- Smith SD, Wheeler MA, Lorber MI, Weiss RM. Temporal changes of cytokines and nitric oxide products in urine from renal transplant patients. Kidney Int. 2000;58:829–837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

