Detecting SARS-CoV-2 at point of care: preliminary data comparing loop-mediated isothermal amplification (LAMP) to polymerase chain reaction (PCR)
- PMID: 33081710
- PMCID: PMC7574392
- DOI: 10.1186/s12879-020-05484-8
Detecting SARS-CoV-2 at point of care: preliminary data comparing loop-mediated isothermal amplification (LAMP) to polymerase chain reaction (PCR)
Abstract
Background: A cost effective and efficient diagnostic tool for COVID-19 as near to the point of care (PoC) as possible would be a game changer in the current pandemic. We tested reverse transcription loop mediated isothermal amplification (RT-LAMP), a method which can produce results in under 30 min, alongside standard methods in a real-life clinical setting.
Methods: This prospective service improvement project piloted an RT-LAMP method on nasal and pharyngeal swabs on 21 residents of a high dependency care home, with two index COVID-19 cases, and compared it to multiplex tandem reverse transcription polymerase chain reaction (RT-PCR). We recorded vital signs of patients to correlate clinical and laboratory information and calculated the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of a single swab using RT-LAMP compared with the current standard, RT-PCR, as per Standards for Reporting Diagnostic Accuracy Studies (STARD) guidelines.
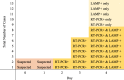
Results: The novel method accurately detected 8/10 RT-PCR positive cases and identified a further 3 positive cases. Eight further cases were negative using both methods. Using repeated RT-PCR as a "gold standard", the sensitivity and specificity of a single novel test were 80 and 73% respectively. PPV was 73% and NPV was 83%. Incorporating retesting of low signal RT-LAMP positives improved the specificity to 100%. We also speculate that hypothermia may be a significant early clinical sign of COVID-19.
Conclusions: RT-LAMP testing for SARS-CoV-2 was found to be promising, fast and to work equivalently to RT-PCR methods. RT-LAMP has the potential to transform COVID-19 detection, bringing rapid and accurate testing to the PoC. RT-LAMP could be deployed in mobile community testing units, care homes and hospitals to detect disease early and prevent spread.
Conflict of interest statement
CJS is supported by HEFCE funding. CS and SW are employees of MicrosensDX Ltd. Testing was provided free of charge by MicrosensDx. Other authors report no conflict of interest.
Figures

References
-
- El-Tholoth M, Bau HH, Song J. A single and two-stage, closed-tube, molecular test for the 2019 novel coronavirus (COVID-19) at home, clinic, and points of entry. ChemRxiv. 2020. 10.26434/chemrxiv.11860137.v1.
-
- Zhang Y, Odiwuor N, Xiong J, et al. Rapid molecular detection of SARS-CoV-2 (COVID-19) virus RNA using colorimetric LAMP. medRxiv. 2020:2020.02.26.20028373. 10.1101/2020.02.26.20028373.
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

