Value of biopsy in a cohort of children with high-titer celiac serologies: observation of dynamic policy differences between Europe and North America
- PMID: 33081760
- PMCID: PMC7576777
- DOI: 10.1186/s12913-020-05815-0
Value of biopsy in a cohort of children with high-titer celiac serologies: observation of dynamic policy differences between Europe and North America
Abstract
Background: Healthcare systems implement change at different rates because of differences in incentives, organizational processes, key influencers, and management styles. A comparable set of forces may play out at the national and international levels as demonstrated in significant differences in the diagnostic management of pediatric Celiac Disease (CD) between European and North American practitioners.
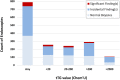
Methods: We use retrospective clinical cohorts of 27,868 serum tissue transglutaminase (tTG) immunoglobulin A levels and 7907 upper gastrointestinal endoscopy pathology reports to create a dataset of 793 pathology reports with matching tTG results between July 1 of 2014 and July 1 of 2018. We use this dataset to characterize histopathological findings in the duodenum, stomach and esophagus of patients as a function of serum tTG levels. In addition, we use the dataset to estimate the local and national cost of endoscopies performed in patients with serum tTG levels greater than 10 times the upper limit of normal.
Results: Using evidence from a US tertiary care center, we show that in the cohort of pediatric patients with high pre-test probability of CD as determined by serum tTG levels, biopsy provides no additional diagnostic value for CD, and that it counter-intuitively introduces diagnostic uncertainty in a number of patients. We estimate that using the European diagnostic algorithms could avoid between 4891 and 7738 pediatric endoscopies per year in the US for evaluation of CD.
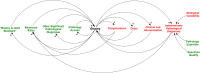
Conclusions: This study considers the North American and European management guidelines for the diagnosis of pediatric CD and highlights the slow adoption in North America of evidence-based algorithms developed and applied in Europe for triage of endoscopy and biopsy. We suggest that system dynamics influences that help maintain the status quo in North America include a variety of social and economic factors in addition to medical evidence. This work contributes to the growing body of evidence that the dynamics that largely favor maintaining status quo management policies in a variety of systems extend to clinical medicine and potentially influence clinical decisions at the level of individual patients and the population.
Keywords: Celiac disease; Health policy; System dynamics; Testing; Value of information.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Evaluation of the ESPGHAN Celiac Guidelines in a North American Pediatric Population.Am J Gastroenterol. 2015 May;110(5):760-7. doi: 10.1038/ajg.2015.87. Epub 2015 Mar 31. Am J Gastroenterol. 2015. PMID: 25823767
-
Validation of Antibody-Based Strategies for Diagnosis of Pediatric Celiac Disease Without Biopsy.Gastroenterology. 2017 Aug;153(2):410-419.e17. doi: 10.1053/j.gastro.2017.04.023. Epub 2017 Apr 28. Gastroenterology. 2017. PMID: 28461188 Clinical Trial.
-
Are ESPGHAN "biopsy-sparing" guidelines for celiac disease also suitable for asymptomatic patients?Am J Gastroenterol. 2015 Oct;110(10):1485-9. doi: 10.1038/ajg.2015.285. Epub 2015 Sep 15. Am J Gastroenterol. 2015. PMID: 26372508
-
Non-biopsy Strategy for the Diagnosis of Celiac Disease in Adults: A Narrative Review.Turk J Gastroenterol. 2024 Aug 2;35(8):589-598. doi: 10.5152/tjg.2024.24092. Turk J Gastroenterol. 2024. PMID: 39150308 Free PMC article. Review.
-
AGA Clinical Practice Update on Diagnosis and Monitoring of Celiac Disease-Changing Utility of Serology and Histologic Measures: Expert Review.Gastroenterology. 2019 Mar;156(4):885-889. doi: 10.1053/j.gastro.2018.12.010. Epub 2018 Dec 19. Gastroenterology. 2019. PMID: 30578783 Free PMC article. Review.
Cited by
-
Celiac Disease: Beyond Diet and Food Awareness.Foods. 2025 Jan 24;14(3):377. doi: 10.3390/foods14030377. Foods. 2025. PMID: 39941971 Free PMC article. Review.
-
Rapid Anti-tTG-IgA Screening Test for Early Diagnosis of Celiac Disease in Pediatric Populations.Nutrients. 2023 Nov 26;15(23):4926. doi: 10.3390/nu15234926. Nutrients. 2023. PMID: 38068784 Free PMC article.
References
-
- Pisano GP, Bohmer RMJ, Edmondson AC. Organizational differences in rates of learning: evidence from the adoption of minimally invasive cardiac surgery. Manag Sci. 2001;47(6):752–768. doi: 10.1287/mnsc.47.6.752.9811. - DOI
-
- Christensen HB, Floyd E, Maffett M. The only prescription is transparency: The effect of charge-price-transparency regulation on healthcare prices. Manage Sci. 2020;66(7):2861-82. 10.1287/mnsc.2019.3330.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

