Amphetamine-like Neurochemical and Cardiovascular Effects of α-Ethylphenethylamine Analogs Found in Dietary Supplements
- PMID: 33082158
- PMCID: PMC7788351
- DOI: 10.1124/jpet.120.000129
Amphetamine-like Neurochemical and Cardiovascular Effects of α-Ethylphenethylamine Analogs Found in Dietary Supplements
Abstract
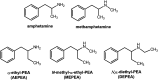
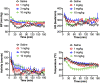
Dietary supplements often contain additives not listed on the label, including α-ethyl homologs of amphetamine such as N,α-diethylphenethylamine (DEPEA). Here, we examined the neurochemical and cardiovascular effects of α-ethylphenethylamine (AEPEA), N-methyl-α-ethylphenethylamine (MEPEA), and DEPEA as compared with the effects of amphetamine. All drugs were tested in vitro using uptake inhibition and release assays for monoamine transporters. As expected, amphetamine acted as a potent and efficacious releasing agent at dopamine transporters (DAT) and norepinephrine transporters (NET) in vitro. AEPEA and MEPEA were also releasers at catecholamine transporters, with greater potency at NET than DAT. DEPEA displayed fully efficacious release at NET but weak partial release at DAT (i.e., 40% of maximal effect). In freely moving, conscious male rats fitted with biotelemetry transmitters for physiologic monitoring, amphetamine (0.1-3.0 mg/kg, s.c.) produced robust dose-related increases in blood pressure (BP), heart rate (HR), and motor activity. AEPEA (1-10 mg/kg, s.c.) produced significant increases in BP but not HR or activity, whereas DEPEA and MEPEA (1-10 mg/kg, s.c.) increased BP, HR, and activity. In general, the phenethylamine analogs were approximately 10-fold less potent than amphetamine. Our results show that α-ethylphenethylamine analogs are biologically active. Although less potent than amphetamine, they produce cardiovascular effects that could pose risks to humans. Given that MEPEA and DEPEA increased locomotor activity, these substances may also have significant abuse potential. SIGNIFICANCE STATEMENT: The α-ethyl homologs of amphetamine have significant cardiovascular, behavioral, and neurochemical effects in rats. Given that these compounds are often not listed on the ingredient labels of dietary supplements, these compounds could pose a risk to humans using these products.
U.S. Government work not protected by U.S. copyright.
Figures



Similar articles
-
Inhibition of monoamine oxidase (MAO) by α-ethylphenethylamine and N,α-diethylphenethylamine, two compounds related to dietary supplements.Food Chem Toxicol. 2014 Dec;74:265-9. doi: 10.1016/j.fct.2014.10.009. Food Chem Toxicol. 2014. PMID: 25455893
-
Reinforcing effects of phenethylamine analogs found in dietary supplements.Psychopharmacology (Berl). 2022 Nov;239(11):3723-3730. doi: 10.1007/s00213-022-06246-x. Epub 2022 Oct 3. Psychopharmacology (Berl). 2022. PMID: 36190536 Free PMC article.
-
The Supplement Adulterant β-Methylphenethylamine Increases Blood Pressure by Acting at Peripheral Norepinephrine Transporters.J Pharmacol Exp Ther. 2019 Jun;369(3):328-336. doi: 10.1124/jpet.118.255976. Epub 2019 Mar 21. J Pharmacol Exp Ther. 2019. PMID: 30898867 Free PMC article.
-
Pharmacology of MDMA- and Amphetamine-Like New Psychoactive Substances.Handb Exp Pharmacol. 2018;252:143-164. doi: 10.1007/164_2018_113. Handb Exp Pharmacol. 2018. PMID: 29633178 Review.
-
New insights into the mechanism of action of amphetamines.Annu Rev Pharmacol Toxicol. 2007;47:681-98. doi: 10.1146/annurev.pharmtox.47.120505.105140. Annu Rev Pharmacol Toxicol. 2007. PMID: 17209801 Review.
Cited by
-
α-Ethyltryptamine: A Ratiocinatory Review of a Forgotten Antidepressant.ACS Pharmacol Transl Sci. 2023 Nov 7;6(12):1780-1789. doi: 10.1021/acsptsci.3c00139. eCollection 2023 Dec 8. ACS Pharmacol Transl Sci. 2023. PMID: 38093842 Free PMC article. Review.
-
Toxicological evaluation, postmortem case descriptions, and pharmacological activity of N,N-dimethylpentylone and related analogs.J Anal Toxicol. 2025 Mar 10;49(3):143-151. doi: 10.1093/jat/bkaf002. J Anal Toxicol. 2025. PMID: 39865839
-
Rapid, open-source, and automated quantification of the head twitch response in C57BL/6J mice using DeepLabCut and Simple Behavioral Analysis.bioRxiv [Preprint]. 2025 Jul 16:2025.04.28.650242. doi: 10.1101/2025.04.28.650242. bioRxiv. 2025. Update in: ACS Pharmacol Transl Sci. 2025 Jul 31;8(8):2694-2709. doi: 10.1021/acsptsci.5c00305. PMID: 40568111 Free PMC article. Updated. Preprint.
-
The psychostimulant drug, fenethylline (captagon): Health risks, addiction and the global impact of illicit trade.Drug Alcohol Depend Rep. 2025 Mar 1;15:100323. doi: 10.1016/j.dadr.2025.100323. eCollection 2025 Jun. Drug Alcohol Depend Rep. 2025. PMID: 40151181 Free PMC article. Review.
-
Automated Computer Software Assessment of 5-Hydroxytryptamine 2A Receptor-Mediated Head Twitch Responses from Video Recordings of Mice.ACS Pharmacol Transl Sci. 2022 Apr 8;5(5):321-330. doi: 10.1021/acsptsci.1c00237. eCollection 2022 May 13. ACS Pharmacol Transl Sci. 2022. PMID: 35592434 Free PMC article.
References
-
- Alsufyani HA, Docherty JR. (2015) Direct and indirect cardiovascular actions of cathinone and MDMA in the anaesthetized rat. Eur J Pharmacol 758:142–146. - PubMed
-
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, et al. (2013) Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 38:552–562. - PMC - PubMed
-
- Branch CA, Knuepfer MM. (1992) Adrenergic mechanisms underlying cardiac and vascular responses to cocaine in conscious rats. J Pharmacol Exp Ther 263:742–751. - PubMed
-
- Brauer LH, de Wit H. (1996) Subjective responses to d-amphetamine alone and after pimozide pretreatment in normal, healthy volunteers. Biol Psychiatry 39:26–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

