Natalizumab versus fingolimod for patients with active relapsing-remitting multiple sclerosis: results from REVEAL, a prospective, randomised head-to-head study
- PMID: 33082194
- PMCID: PMC7577060
- DOI: 10.1136/bmjopen-2020-038861
Natalizumab versus fingolimod for patients with active relapsing-remitting multiple sclerosis: results from REVEAL, a prospective, randomised head-to-head study
Abstract
Objective: To directly compare the efficacy of natalizumab and fingolimod in patients with active relapsing-remitting multiple sclerosis.
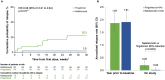
Methods: This phase 4, randomised, rater- and sponsor-blinded, prospective, parallel-group, clinic-based head-to-head study was conducted at 43 sites in nine countries. Patients were randomised (1:1) to intravenous natalizumab 300 mg every 4 weeks or oral fingolimod 0.5 mg once daily for ≤52 weeks. Enrolment-related early study termination precluded assessment of the primary endpoint (evolution of new on-treatment gadolinium-enhancing (Gd+) lesions to persistent black holes). Unplanned exploratory analyses of secondary endpoints evaluated the effects of treatment on the development of new T1 Gd+ lesions and new/newly enlarging T2 lesions, lesion volumes and relapse outcomes.
Results: The intent-to-treat population comprised 108 patients (natalizumab, n=54; fingolimod, n=54); 63 completed ≥24 weeks of treatment. Due to the limited numbers of events and patients at risk, MRI and relapse outcomes were reported over up to 24 and 36 weeks, respectively. The mean number of new T1 Gd+ lesions was numerically lower with natalizumab than with fingolimod by 4 weeks; accumulation rates were 0.02 and 0.09 per week, respectively, over 24 weeks (p=0.004). The cumulative probability of developing ≥1 lesion at 24 weeks was 40.7% with natalizumab versus 58.0% with fingolimod (HR=0.60; 95% CI 0.31-1.16; p=0.126); the corresponding probabilities for ≥2 lesions were 11.5% vs 48.5% (HR=0.25; 95% CI 0.09-0.68; p=0.007). No significant between-group differences were observed for the other MRI outcomes at 24 weeks. The cumulative probability of relapse over follow-up was 1.9% with natalizumab versus 22.3% with fingolimod (HR=0.08; 95% CI 0.01-0.64; p=0.017). Adverse events were consistent with known safety profiles.
Conclusions: These results suggest that natalizumab is more efficacious than fingolimod in reducing multiple sclerosis relapses and T1 Gd+ lesion accumulation in patients with active disease.
Trial registration numbers: NCT02342704; EUCTR2013-004622-29-IT; Post-results.
Keywords: clinical trials; multiple sclerosis; neurology.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: HB has received compensation for consulting from Biogen, Merck Serono and Novartis and research support from Biogen and Merck Serono. SL and NC are employees of and may hold stock and/or stock options in Biogen. DJ has received research funding from Biogen and Genentech and personal compensation for speaking or consulting services from Acorda, Bayer, Biogen, Genentech, GlaxoSmithKline, Novartis, Questcor, Serono and Teva. DLA has served on advisory boards for, received speaker honoraria from, served as a consultant for or received research support from Bayer, Biogen, Coronado Biosciences, the Consortium of Multiple Sclerosis Centers, Eli Lilly, EMD Serono, Genentech, Genzyme, GlaxoSmithKline, Merck Serono, MS Forum, NeuroRx Research, Novartis, Opexa Therapeutics, Roche, Teva, the Canadian Institutes of Health Research, the Multiple Sclerosis Society of Canada and the SA Serono Symposia International Foundation, and he holds stock in NeuroRx Research. MF is editor-in-chief of the Journal of Neurology; has received compensation for consulting services and/or speaking activities from Biogen, Merck Serono, Novartis and Teva; and has received research support from Biogen, Merck Serono, Novartis, Roche, Teva, the Italian Ministry of Health, la Fondazione Italiana Sclerosi Multipla (FISM) and la Fondazione Italiana di Ricerca per la Sclerosi Laterale Amiotrofica (AriSLA). JG serves on the editorial boards of Multiple Sclerosis Journal and Neurology; has received speaker honoraria from Biogen, Genzyme, Merck Serono, Novartis and Teva; has received research support from Biogen; and has served on the boards of the Dutch MS Research Foundation and the Progressive MS Alliance. SS and P-RH were employees of Biogen at the time of these analyses and may hold stock and/or stock options in Biogen.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
