The Relative Expression of ERα Isoforms ERα66 and ERα36 Controls the Cellular Response to 24R,25-Dihydroxyvitamin D3 in Breast Cancer
- PMID: 33082240
- PMCID: PMC9250782
- DOI: 10.1158/1541-7786.MCR-20-0169
The Relative Expression of ERα Isoforms ERα66 and ERα36 Controls the Cellular Response to 24R,25-Dihydroxyvitamin D3 in Breast Cancer
Abstract
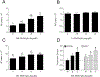
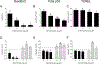
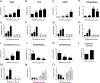
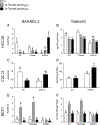
Vitamin D3 and its metabolites have antitumorigenic properties in vitro and in vivo; however, clinical trials and retrospective studies on the effectiveness of vitamin D3 oral supplementation against cancer have been inconclusive. One reason for this may be that clinical trials ignore the complex vitamin D metabolome and the many active vitamin D3 metabolites present in the body. Recent work by our lab showed that 24R,25(OH)2D3, a vitamin D3 metabolite that is active in chondrocyte proliferation and differentiation, has antitumorigenic properties in estrogen receptor alpha-66 (ERα66)-positive (ER+) breast cancer, but not in ERα66-negative (ER-) breast cancer. Here we show that 24R,25(OH)2D3 is protumorigenic in an in vivo mouse model (NOD.Cg-PrkdcscidIl2rgtm1Wjl /SzJ (NSG) mice) of ER- breast cancer, causing greater tumor growth than in mice treated with vehicle alone. In vitro results indicate that the effect of 24R,25(OH)2D3 is via a membrane-associated mechanism involving ERs and phospholipase D. 24R,25(OH)2D3 increased proliferation and reduced apoptosis in ERα66-negative HCC38 breast cancer cells, and stimulated expression of metastatic markers. Overexpressing ESRI, which encodes ERα66, ERα46, and ERα36, reduced the proapoptotic response of ERα66- cells to 24R,25(OH)2D3, possibly by upregulating ERα66. Silencing ESR1 in ERα66+ cells increased apoptosis. This suggests 24R,25(OH)2D3 is differentially tumorigenic in cancers with different ERα isoform profiles. Antiapoptotic actions of 24R,25(OH)2D3 require ERα36 and proapoptotic actions require ERα66. IMPLICATIONS: These results suggest that 24R,25(OH)2D3, which is a major circulating metabolite of vitamin D, is functionally active in breast cancer and that the regulatory properties of 24R,25(OH)2D3 are dependent upon the relative expression of ERα66 and ERα36.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures
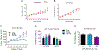
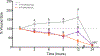

Similar articles
-
Laryngeal Cancer Cells Metabolize 25-Hydroxyvitamin D3 and Respond to 24R,25-dihydroxyvitamin D3 via a Mechanism Dependent on Estrogen Receptor Levels.Cancers (Basel). 2024 Apr 24;16(9):1635. doi: 10.3390/cancers16091635. Cancers (Basel). 2024. PMID: 38730587 Free PMC article.
-
24R,25-Dihydroxyvitamin D3 regulates breast cancer cells in vitro and in vivo.Biochim Biophys Acta Gen Subj. 2019 Oct;1863(10):1498-1512. doi: 10.1016/j.bbagen.2019.05.013. Epub 2019 May 22. Biochim Biophys Acta Gen Subj. 2019. PMID: 31125679
-
Local production of active vitamin D3 metabolites in breast cancer cells by CYP24A1 and CYP27B1.J Steroid Biochem Mol Biol. 2023 Sep;232:106331. doi: 10.1016/j.jsbmb.2023.106331. Epub 2023 May 25. J Steroid Biochem Mol Biol. 2023. PMID: 37244301
-
24R,25-dihydroxyvitamin D3 modulates tumorigenicity in breast cancer in an estrogen receptor-dependent manner.Steroids. 2019 Oct;150:108447. doi: 10.1016/j.steroids.2019.108447. Epub 2019 Jul 11. Steroids. 2019. PMID: 31302113 Review.
-
Vitamin D and systemic cancer: is this relevant to malignant melanoma?Br J Dermatol. 2002 Aug;147(2):197-213. doi: 10.1046/j.1365-2133.2002.04960.x. Br J Dermatol. 2002. PMID: 12174089 Review.
Cited by
-
Laryngeal Cancer Cells Metabolize 25-Hydroxyvitamin D3 and Respond to 24R,25-dihydroxyvitamin D3 via a Mechanism Dependent on Estrogen Receptor Levels.Cancers (Basel). 2024 Apr 24;16(9):1635. doi: 10.3390/cancers16091635. Cancers (Basel). 2024. PMID: 38730587 Free PMC article.
-
Impact of estrogen receptor expression on prognosis of ovarian cancer according to antibody clone used for immunohistochemistry: a meta-analysis.J Ovarian Res. 2022 May 24;15(1):63. doi: 10.1186/s13048-022-01001-4. J Ovarian Res. 2022. PMID: 35610648 Free PMC article.
-
Vitamin D, Th17 Lymphocytes, and Breast Cancer.Cancers (Basel). 2022 Jul 27;14(15):3649. doi: 10.3390/cancers14153649. Cancers (Basel). 2022. PMID: 35954312 Free PMC article. Review.
-
Key genes of vitamin D metabolism and their roles in the risk and prognosis of cancer.Front Genet. 2025 Jun 24;16:1598525. doi: 10.3389/fgene.2025.1598525. eCollection 2025. Front Genet. 2025. PMID: 40630116 Free PMC article. Review.
-
Synthesis of Deuterium-Labeled Vitamin D Metabolites as Internal Standards for LC-MS Analysis.Molecules. 2022 Apr 9;27(8):2427. doi: 10.3390/molecules27082427. Molecules. 2022. PMID: 35458625 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

