A Giant Extracellular Matrix Binding Protein of Staphylococcus epidermidis Binds Surface-Immobilized Fibronectin via a Novel Mechanism
- PMID: 33082256
- PMCID: PMC7587433
- DOI: 10.1128/mBio.01612-20
A Giant Extracellular Matrix Binding Protein of Staphylococcus epidermidis Binds Surface-Immobilized Fibronectin via a Novel Mechanism
Abstract
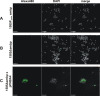
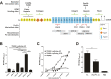
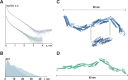
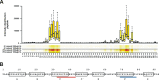
Although it is normally an innocuous part of the human skin microbiota, Staphylococcus epidermidis has emerged as a major nosocomial pathogen, and implanted foreign materials are an essential risk factor for the development of an infection. The extraordinary efficiency of S. epidermidis to colonize artificial surfaces is particularly related to the ability to form biofilms. Biofilm formation itself critically depends on stable pathogen binding to extracellular host matrix components, e.g. fibronectin (Fn), covering inserted devices in vast amounts. Extracellular matrix binding protein (Embp) and its subdomains referred to as the F-repeat and the FG-repeat are critical for adherence of S. epidermidis to surface-immobilized Fn. Embp-Fn interactions preferentially occur with surface-bound, but not folded, globular Fn via binding to the F3 domain. High-resolution structure analysis of F- and FG-repeats revealed that both repeats are composed of two tightly connected triple α-helix bundles, exhibiting an elongated but rather rigid structural organization in solution. Both F- and FG-repeat possess Fn-binding capacity via interactions with type III subdomain FN12, involving residues within the C and F β-sheet. FN12 essentially supports stability of the globular Fn state, and thus these findings reasonably explain why Embp-mediated interaction of S. epidermidis necessitates Fn surface immobilization. Thus, Embp employs an uncharacterized bacterial Fn-binding mechanism to promote staphylococcal adherence.IMPORTANCEStaphylococcus epidermidis is a leading pathogen in implant-associated hospital infections. The pathogenesis critically depends on bacterial binding to ECM components, specifically fibronectin (Fn). The cell surface-localized, 1-MDa extracellular matrix binding protein (Embp) is essentially characterized by 10 F- and 40 FG-repeats. These repetitive units, each characterized by two α-helical bundles, organize themselves in a rigid, elongated form. Embp binds preferentially to surface-localized but not soluble Fn, with both F- and FG-repeats being sufficient for Fn binding and resulting bacterial adherence. Binding preferentially involves Fn type III domain, specifically residues of FN12 β-sheets C and F. Both play key role in stabilizing the globular Fn conformation, explaining the necessity of Fn surface immobilization for a subsequent interaction with Embp. In comparison to many other bacterial Fn-binding proteins using the Fn N terminus, Embp employs a previously undescribed mechanism supporting the adhesion of S. epidermidis to surface-immobilized Fn.
Keywords: Staphylococcus; biofilms; fibronectin binding; surface proteins; surface structures.
Copyright © 2020 Büttner et al.
Figures



Similar articles
-
The giant staphylococcal protein Embp facilitates colonization of surfaces through Velcro-like attachment to fibrillated fibronectin.Elife. 2022 Jul 7;11:e76164. doi: 10.7554/eLife.76164. Elife. 2022. PMID: 35796649 Free PMC article.
-
The giant extracellular matrix-binding protein of Staphylococcus epidermidis mediates biofilm accumulation and attachment to fibronectin.Mol Microbiol. 2010 Jan;75(1):187-207. doi: 10.1111/j.1365-2958.2009.06981.x. Epub 2009 Nov 25. Mol Microbiol. 2010. PMID: 19943904
-
Host factors abolish the need for polysaccharides and extracellular matrix-binding protein in Staphylococcus epidermidis biofilm formation.J Med Microbiol. 2021 Mar;70(3):001287. doi: 10.1099/jmm.0.001287. Epub 2021 Jan 22. J Med Microbiol. 2021. PMID: 33492206 Free PMC article.
-
Protein-based biofilm matrices in Staphylococci.Front Cell Infect Microbiol. 2014 Dec 10;4:171. doi: 10.3389/fcimb.2014.00171. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25540773 Free PMC article. Review.
-
Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells.Matrix Biol. 1999 Jun;18(3):211-23. doi: 10.1016/s0945-053x(99)00025-6. Matrix Biol. 1999. PMID: 10429941 Review.
Cited by
-
Staphylococcus epidermidis alters macrophage polarization and phagocytic uptake by extracellular DNA release in vitro.NPJ Biofilms Microbiomes. 2024 Nov 20;10(1):131. doi: 10.1038/s41522-024-00604-7. NPJ Biofilms Microbiomes. 2024. PMID: 39567551 Free PMC article.
-
The giant staphylococcal protein Embp facilitates colonization of surfaces through Velcro-like attachment to fibrillated fibronectin.Elife. 2022 Jul 7;11:e76164. doi: 10.7554/eLife.76164. Elife. 2022. PMID: 35796649 Free PMC article.
-
Staphylococcus epidermidis and its dual lifestyle in skin health and infection.Nat Rev Microbiol. 2023 Feb;21(2):97-111. doi: 10.1038/s41579-022-00780-3. Epub 2022 Aug 30. Nat Rev Microbiol. 2023. PMID: 36042296 Free PMC article. Review.
-
Glaesserella parasuis serotype 4 exploits fibronectin via RlpA for tracheal colonization following porcine circovirus type 2 infection.PLoS Pathog. 2024 Sep 12;20(9):e1012513. doi: 10.1371/journal.ppat.1012513. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39264911 Free PMC article.
-
Monoclonal Antibodies Targeting Surface-Exposed and Secreted Proteins from Staphylococci.Vaccines (Basel). 2021 May 4;9(5):459. doi: 10.3390/vaccines9050459. Vaccines (Basel). 2021. PMID: 34064471 Free PMC article. Review.
References
-
- Schommer NN, Christner M, Hentschke M, Ruckdeschel K, Aepfelbacher M, Rohde H. 2011. Staphylococcus epidermidis uses distinct mechanisms of biofilm formation to interfere with phagocytosis and activation of mouse macrophage-like cells 774A.1. Infect Immun 79:2267–2276. doi:10.1128/IAI.01142-10. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
