The ZIP Code of Vesicle Trafficking in Apicomplexa: SEC1/Munc18 and SNARE Proteins
- PMID: 33082261
- PMCID: PMC7587439
- DOI: 10.1128/mBio.02092-20
The ZIP Code of Vesicle Trafficking in Apicomplexa: SEC1/Munc18 and SNARE Proteins
Abstract
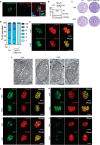
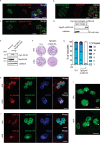
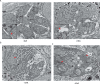
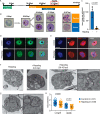
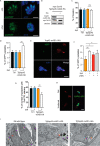
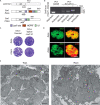
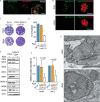
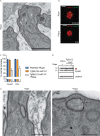
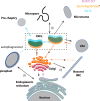
Apicomplexans are obligate intracellular parasites harboring three sets of unique secretory organelles termed micronemes, rhoptries, and dense granules that are dedicated to the establishment of infection in the host cell. Apicomplexans rely on the endolysosomal system to generate the secretory organelles and to ingest and digest host cell proteins. These parasites also possess a metabolically relevant secondary endosymbiotic organelle, the apicoplast, which relies on vesicular trafficking for correct incorporation of nuclear-encoded proteins into the organelle. Here, we demonstrate that the trafficking and destination of vesicles to the unique and specialized parasite compartments depend on SNARE proteins that interact with tethering factors. Specifically, all secreted proteins depend on the function of SLY1 at the Golgi. In addition to a critical role in trafficking of endocytosed host proteins, TgVps45 is implicated in the biogenesis of the inner membrane complex (alveoli) in both Toxoplasma gondii and Plasmodium falciparum, likely acting in a coordinated manner with Stx16 and Stx6. Finally, Stx12 localizes to the endosomal-like compartment and is involved in the trafficking of proteins to the apical secretory organelles rhoptries and micronemes as well as to the apicoplast.IMPORTANCE The phylum of Apicomplexa groups medically relevant parasites such as those responsible for malaria and toxoplasmosis. As members of the Alveolata superphylum, these protozoans possess specialized organelles in addition to those found in all members of the eukaryotic kingdom. Vesicular trafficking is the major route of communication between membranous organelles. Neither the molecular mechanism that allows communication between organelles nor the vesicular fusion events that underlie it are completely understood in Apicomplexa. Here, we assessed the function of SEC1/Munc18 and SNARE proteins to identify factors involved in the trafficking of vesicles between these various organelles. We show that SEC1/Munc18 in interaction with SNARE proteins allows targeting of vesicles to the inner membrane complex, prerhoptries, micronemes, apicoplast, and vacuolar compartment from the endoplasmic reticulum, Golgi apparatus, or endosomal-like compartment. These data provide an exciting look at the "ZIP code" of vesicular trafficking in apicomplexans, essential for precise organelle biogenesis, homeostasis, and inheritance.
Keywords: Apicomplexa; Plasmodium falciparum; SEC1/Munc18; SNARE; Toxoplasma gondii; Vps45; apicomplexan parasites; apicoplast; exocytosis; inner membrane complex; membrane fusion; microneme; pellicle; rhoptry; small GTPases; syntaxin.
Copyright © 2020 Bisio et al.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
