Antibody Neutralization of HIV-1 Crossing the Blood-Brain Barrier
- PMID: 33082263
- PMCID: PMC7587443
- DOI: 10.1128/mBio.02424-20
Antibody Neutralization of HIV-1 Crossing the Blood-Brain Barrier
Abstract
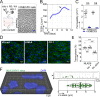
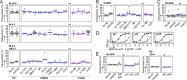
HIV-1 can cross the blood-brain barrier (BBB) to penetrate the brain and infect target cells, causing neurocognitive disorders as a result of neuroinflammation and brain damage. Here, we examined whether antibodies targeting the HIV-1 envelope glycoproteins interfere with the transcytosis of virions across the human BBB endothelium. We found that although the viral envelope spike gp160 is required for optimal endothelial cell endocytosis, no anti-gp160 antibodies blocked the BBB transcytosis of HIV-1 in vitro Instead, both free viruses and those in complex with antibodies transited across endothelial cells in the BBB model, as observed by confocal microscopy. HIV-1 infectious capacity was considerably altered by the transcytosis process but still detectable, even in the presence of nonneutralizing antibodies. Only virions bound by neutralizing antibodies lacked posttranscytosis infectivity. Overall, our data support the role of neutralizing antibodies in protecting susceptible brain cells from HIV-1 infection despite their inability to inhibit viral BBB endocytic transport.IMPORTANCE HIV-1 can cross the blood-brain barrier (BBB) to penetrate the brain and infect target cells, causing neurocognitive disorders as a result of neuroinflammation and brain damage. The HIV-1 envelope spike gp160 is partially required for viral transcytosis across the BBB endothelium. But do antibodies developing in infected individuals and targeting the HIV-1 gp160 glycoproteins block HIV-1 transcytosis through the BBB? We addressed this issue and discovered that anti-gp160 antibodies do not block HIV-1 transport; instead, free viruses and those in complex with antibodies can transit across BBB endothelial cells. Importantly, we found that only neutralizing antibodies could inhibit posttranscytosis viral infectivity, highlighting their ability to protect susceptible brain cells from HIV-1 infection.
Keywords: HIV-1; antibodies; blood brain barrier; neutralization; transcytosis.
Copyright © 2020 Lorin et al.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
