Accurate Diagnosis of COVID-19 by a Novel Immunogenic Secreted SARS-CoV-2 orf8 Protein
- PMID: 33082264
- PMCID: PMC7587431
- DOI: 10.1128/mBio.02431-20
Accurate Diagnosis of COVID-19 by a Novel Immunogenic Secreted SARS-CoV-2 orf8 Protein
Abstract
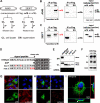
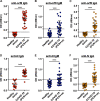
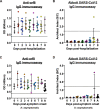
An accurate diagnostic test for early severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is the key weapon to control the coronavirus disease 2019 (COVID-19) pandemic. We previously reported that the SARS-CoV-2 genome contains a unique orf8 accessory gene absent from other human-pathogenic coronaviruses. Here, we characterized the SARS-CoV-2 orf8 as a novel immunogenic secreted protein and utilized it for the accurate diagnosis of COVID-19. Extracellular orf8 protein was detected in cell culture supernatant and in sera of COVID-19 patients. In addition, orf8 was found highly immunogenic in COVID-19 patients, who showed early seropositivity for anti-orf8 IgM, IgG, and IgA. We hypothesize that orf8 secretion during SARS-CoV-2 infection facilitates early mounting of B cell response. The serological test detecting anti-orf8 IgG antibody can be used for the early and accurate diagnosis of COVID-19.IMPORTANCE Current commercially available serological tests for COVID-19 patients are detecting antibodies against SARS-CoV-2 nucleoprotein and spike glycoprotein. The antinucleoprotein and antispike antibodies can be accurately detected in patients during the mid or late stage of infection, and therefore, these assays have not been widely used for early diagnosis of COVID-19. In this study, we characterized the secretory property of a SARS-CoV-2 orf8 protein and proposed that orf8 secretion during infection facilitates early mounting of the B cell response. We demonstrated the presence of anti-orf8 antibodies in both symptomatic and asymptomatic patients during the early stage of infection, while the anti-N antibody is not detected. Our serological test detecting anti-orf8 antibodies may facilitate the development of early and accurate diagnosis for COVID-19.
Keywords: COVID-19; SARS-CoV-2; diagnosis; orf8.
Copyright © 2020 Wang et al.
Figures



References
-
- World Health Organization. 2020. WHO coronavirus disease (COVID-19) dashboard. World Health Organization, Geneva, Switzerland: https://covid19.who.int/.
-
- Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, Jiang K, Arunkumar GA, Jurczyszak D, Polanco J, Bermudez-Gonzalez M, Kleiner G, Aydillo T, Miorin L, Fierer DS, Lugo LA, Kojic EM, Stoever J, Liu STH, Cunningham-Rundles C, Felgner PL, Moran T, Garcia-Sastre A, Caplivski D, Cheng AC, Kedzierska K, Vapalahti O, Hepojoki JM, Simon V, Krammer F. 2020. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 26:1033–1036. doi: 10.1038/s41591-020-0913-5. - DOI - PMC - PubMed
-
- Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, Hong C, Wang F, Liu Y, Wang Z, He Q, Li Z, He B, Zhang T, Fu Y, Ge S, Liu L, Zhang J, Xia N, Zhang Z. 2020. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis 28:ciaa344. doi: 10.1093/cid/ciaa344. - DOI - PMC - PubMed
-
- To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, Yip CC, Cai JP, Chan JM, Chik TS, Lau DP, Choi CY, Chen LL, Chan WM, Chan KH, Ip JD, Ng AC, Poon RW, Luo CT, Cheng VC, Chan JF, Hung IF, Chen Z, Chen H, Yuen KY. 2020. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 20:565–574. doi: 10.1016/S1473-3099(20)30196-1. - DOI - PMC - PubMed
-
- Reference deleted.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
