Effect of IBD medications on COVID-19 outcomes: results from an international registry
- PMID: 33082265
- PMCID: PMC8136807
- DOI: 10.1136/gutjnl-2020-322539
Effect of IBD medications on COVID-19 outcomes: results from an international registry
Abstract
Objective: We sought to evaluate COVID-19 clinical course in patients with IBD treated with different medication classes and combinations.
Design: Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) is a large, international registry created to monitor outcomes of IBD patients with confirmed COVID-19. We used multivariable regression with a generalised estimating equation accounting for country as a random effect to analyse the association of different medication classes with severe COVID-19, defined as intensive care unit admission, ventilator use and/or death.
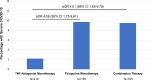
Results: 1439 cases from 47 countries were included (mean age 44.1 years, 51.4% men) of whom 112 patients (7.8%) had severe COVID-19. Compared with tumour necrosis factor (TNF) antagonist monotherapy, thiopurine monotherapy (adjusted OR (aOR) 4.08, 95% CI 1.73 to 9.61) and combination therapy with TNF antagonist and thiopurine (aOR 4.01, 95% CI 1.65 to 9.78) were associated with an increased risk of severe COVID-19. Any mesalamine/sulfasalazine compared with no mesalamine/sulfasalazine use was associated with an increased risk (aOR 1.70, 95% CI 1.26 to 2.29). This risk estimate increased when using TNF antagonist monotherapy as a reference group (aOR 3.52, 95% CI 1.93 to 6.45). Interleukin-12/23 and integrin antagonists were not associated with significantly different risk than TNF antagonist monotherapy (aOR 0.98, 95% CI 0.12 to 8.06 and aOR 2.42, 95% CI 0.59 to 9.96, respectively).
Conclusion: Combination therapy and thiopurines may be associated with an increased risk of severe COVID-19. No significant differences were observed when comparing classes of biologicals. These findings warrant confirmation in large population-based cohorts.MKH should be changed to MDK for co-last author line.
Keywords: infectious disease; inflammatory bowel disease.
© Author(s) (or their employer(s)) 2021. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RCU has served as an advisory board member or consultant for Eli Lilly, Janssen, Pfizer and Takeda; research support from AbbVie, Boehringer Ingelheim and Pfizer. EB: no conflicts of interest. RG: speaker fees and Scientific Advisory Boards for AbbVie and Janssen. GGK: honoraria for speaking or consultancy from Abbvie, Janssen, Pfizer and Takeda. He has received research support from Ferring, Janssen, Abbvie, GlaxoSmith Kline, Merck and Shire. Ownership of a patent: treatment of inflammatory disorders, autoimmune disease, and PBC. UTI Limited Partnership, assignee. Patent WO2019046959A1. PCT/CA2018/051098. 7 September 2018. MK-H: speaker/consultant for AbbVie, Janssen and Takeda. JDL: personal fees from Johnson & Johnson Consumer Inc; grants, personal fees and other from Takeda Pharmaceuticals; personal fees and non-financial support from AbbVie; grants and personal fees from Janssen Pharmaceuticals; personal fees from Eli Lilly and Company; personal fees from Samsung Bioepis; personal fees from UCB; personal fees from Bristol-Myers Squibb; grants and personal fees from Nestle Health Science; personal fees from Bridge Biotherapeutics; personal fees from Celgene; personal fees from Merck; personal fees and other from Pfizer; personal fees from Gilead; personal fees from Arena Parmaceuticals; and personal fees from Protagonist Therapeutics. SCN: honoraria for speaking or consultancy from Abbvie, Janssen, Ferring, Tillotts and Takeda; research support from Ferring and AbbVie. WR has served as a speaker for Abbott Laboratories, Abbvie, Aesca, Aptalis, Astellas, Centocor, Celltrion, Danone Austria, Elan, Falk Pharma GmbH, Ferring, Immundiagnostik, Mitsubishi Tanabe Pharma Corporation, MSD, Otsuka, PDL, Pharmacosmos, PLS Education, Schering-Plough, Shire, Takeda, Therakos, Vifor and Yakult. He has been a consultant for Abbott Laboratories, Abbvie, Aesca, Algernon, Amgen, AM Pharma, AMT, AOP Orphan, Arena Pharmaceuticals, Astellas, Astra Zeneca, Avaxia, Roland Berger GmBH, Bioclinica, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Covance, Danone Austria, DSM, Elan, Eli Lilly, Ernest & Young, Falk Pharma GmbH, Ferring, Galapagos, Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Intrinsic Imaging, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, LivaNova, Mallinckrodt, Medahead, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nash Pharmaceuticals, Nestle, Nippon Kayaku, Novartis, Ocera, OMass, Otsuka, Parexel, PDL, Periconsulting, Pharmacosmos, Philip Morris Institute, Pfizer, Procter & Gamble, Prometheus, Protagonist, Provention, Robarts Clinical Trial, Sandoz, Schering-Plough, Second Genome, Seres Therapeutics, Setpointmedical, Sigmoid, Sublimity, Takeda, Therakos, Theravance, Tigenix, UCB, Vifor, Zealand, Zyngenia and 4SC. He has been as an advisory board member for Abbott Laboratories, Abbvie, Aesca, Amgen, AM Pharma, Astellas, Astra Zeneca, Avaxia, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Danone Austria, DSM, Elan, Ferring, Galapagos, Genentech, Grünenthal, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestle, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Sandoz, Schering-Plough, Second Genome, Setpointmedical, Takeda, Therakos, Tigenix, UCB, Zealand, Zyngenia and 4SC. He has received research funding from Abbott Laboratories, Abbvie, Aesca, Centocor, Falk Pharma GmbH, Immundiagnsotik and MSD. FR: consultation fee, research grant or honorarium from Janssen, Pfizer, Abbvie, Takeda, Celgene, Nestlé Health Science and Nestlé Nutrition Institute. FS: speaker and consultant for: AbbVie, Eurofarma, Ferring, Janssen, Pfizer, Sanofi, Takeda and UCB. FEU: no conflicts of interest. XZ: no conflicts of interest. J-FC: research grants from AbbVie, Janssen Pharmaceuticals and Takeda; receiving payment for lectures from AbbVie, Amgen, Allergan, Inc, Ferring Pharmaceuticals, Shire and Takeda; receiving consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene Corporation, Celltrion, Eli Lilly, Enterome, Ferring Pharmaceuticals, Genentech, Janssen Pharmaceuticals, Landos, Ipsen, Medimmune, Merck, Novartis, Pfizer, Shire, Takeda, Tigenix and Viela bio; and hold stock options in Intestinal Biotech Development and Genfit. MK has consulted for AbbVie, Janssen and Takeda, is a shareholder in Johnson & Johnson and has received research support from AbbVie and Janssen.
Figures
Comment in
-
COVID-19 and IBD drugs: should we change anything at the moment?Gut. 2021 Apr;70(4):632-634. doi: 10.1136/gutjnl-2020-323247. Epub 2020 Nov 19. Gut. 2021. PMID: 33214164 No abstract available.
-
COVID-19 and Inflammatory Bowel Disease: Lessons Learned, Practical Recommendations, and Unanswered Questions.Gastroenterology. 2021 Apr;160(5):1447-1451. doi: 10.1053/j.gastro.2020.12.042. Epub 2020 Dec 30. Gastroenterology. 2021. PMID: 33387525 Free PMC article. No abstract available.
-
Maintenance therapy with infliximab or vedolizumab in IBD is not associated with increased SARS-CoV-2 seroprevalence: UK experience in the 2020 pandemic.Gut. 2021 Dec;70(12):2398-2400. doi: 10.1136/gutjnl-2021-324116. Epub 2021 Feb 12. Gut. 2021. PMID: 33579788 No abstract available.
-
Association between 5-aminosalicylates in patients with IBD and risk of severe COVID-19: an artefactual result of research methodology?Gut. 2021 Oct;70(10):2020-2022. doi: 10.1136/gutjnl-2021-324397. Epub 2021 Mar 3. Gut. 2021. PMID: 33658323 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
