Interclass GPCR heteromerization affects localization and trafficking
- PMID: 33082287
- PMCID: PMC7717648
- DOI: 10.1126/scisignal.aaw3122
Interclass GPCR heteromerization affects localization and trafficking
Abstract
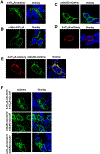
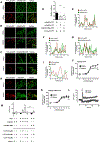
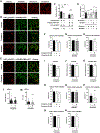
Membrane trafficking processes regulate G protein-coupled receptor (GPCR) activity. Although class A GPCRs are capable of activating G proteins in a monomeric form, they can also potentially assemble into functional GPCR heteromers. Here, we showed that the class A serotonin 5-HT2A receptors (5-HT2ARs) affected the localization and trafficking of class C metabotropic glutamate receptor 2 (mGluR2) through a mechanism that required their assembly as heteromers in mammalian cells. In the absence of agonists, 5-HT2AR was primarily localized within intracellular compartments, and coexpression of 5-HT2AR with mGluR2 increased the intracellular distribution of the otherwise plasma membrane-localized mGluR2. Agonists for either 5-HT2AR or mGluR2 differentially affected trafficking through Rab5-positive endosomes in cells expressing each component of the 5-HT2AR-mGluR2 heterocomplex alone, or together. In addition, overnight pharmacological 5-HT2AR blockade with clozapine, but not with M100907, decreased mGluR2 density through a mechanism that involved heteromerization between 5-HT2AR and mGluR2. Using TAT-tagged peptides and chimeric constructs that are unable to form the interclass 5-HT2AR-mGluR2 complex, we demonstrated that heteromerization was necessary for the 5-HT2AR-dependent effects on mGluR2 subcellular distribution. The expression of 5-HT2AR also augmented intracellular localization of mGluR2 in mouse frontal cortex pyramidal neurons. Together, our data suggest that GPCR heteromerization may itself represent a mechanism of receptor trafficking and sorting.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

