Toxic oligomers of the amyloidogenic HypF-N protein form pores in mitochondrial membranes
- PMID: 33082392
- PMCID: PMC7575562
- DOI: 10.1038/s41598-020-74841-z
Toxic oligomers of the amyloidogenic HypF-N protein form pores in mitochondrial membranes
Abstract
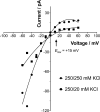
Studies on the amyloidogenic N-terminal domain of the E. coli HypF protein (HypF-N) have contributed significantly to a detailed understanding of the pathogenic mechanisms in neurodegenerative diseases characterised by the formation of misfolded oligomers, by proteins such as amyloid-β, α-synuclein and tau. Given that both cell membranes and mitochondria are increasingly recognised as key targets of oligomer toxicity, we investigated the damaging effects of aggregates of HypF-N on mitochondrial membranes. Essentially, we found that HypF-N oligomers characterised by high surface hydrophobicity (type A) were able to trigger a robust permeabilisation of mito-mimetic liposomes possessing cardiolipin-rich membranes and dysfunction of isolated mitochondria, as demonstrated by a combination of mitochondrial shrinking, lowering of mitochondrial membrane potential and cytochrome c release. Furthermore, using single-channel electrophysiology recordings we obtained evidence that the type A aggregates induced currents reflecting formation of ion-conducting pores in mito-mimetic planar phospholipid bilayers, with multi-level conductances ranging in the hundreds of pS at negative membrane voltages. Conversely, HypF-N oligomers with low surface hydrophobicity (type B) could not permeabilise or porate mitochondrial membranes. These results suggest an inherent toxicity of membrane-active aggregates of amyloid-forming proteins to mitochondria, and that targeting of oligomer-mitochondrial membrane interactions might therefore afford protection against such damage.
Conflict of interest statement
The authors declare no competing interests.
Figures





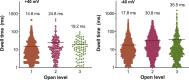
Similar articles
-
Cardiolipin Promotes Pore-Forming Activity of Alpha-Synuclein Oligomers in Mitochondrial Membranes.ACS Chem Neurosci. 2019 Aug 21;10(8):3815-3829. doi: 10.1021/acschemneuro.9b00320. Epub 2019 Aug 6. ACS Chem Neurosci. 2019. PMID: 31356747
-
Mitochondrial membrane permeabilisation by amyloid aggregates and protection by polyphenols.Biochim Biophys Acta. 2013 Nov;1828(11):2532-43. doi: 10.1016/j.bbamem.2013.06.026. Epub 2013 Jun 28. Biochim Biophys Acta. 2013. PMID: 23817009
-
Glycosaminoglycans (GAGs) suppress the toxicity of HypF-N prefibrillar aggregates.J Mol Biol. 2012 Aug 24;421(4-5):616-30. doi: 10.1016/j.jmb.2012.02.007. Epub 2012 Feb 9. J Mol Biol. 2012. PMID: 22326346
-
Poration of mitochondrial membranes by amyloidogenic peptides and other biological toxins.J Neurochem. 2025 Jan;169(1):e16213. doi: 10.1111/jnc.16213. Epub 2024 Aug 30. J Neurochem. 2025. PMID: 39213385 Review.
-
Amyloid β, α-synuclein and the c subunit of the ATP synthase: Can these peptides reveal an amyloidogenic pathway of the permeability transition pore?Biochim Biophys Acta Biomembr. 2021 Mar 1;1863(3):183531. doi: 10.1016/j.bbamem.2020.183531. Epub 2020 Dec 10. Biochim Biophys Acta Biomembr. 2021. PMID: 33309700 Free PMC article. Review.
Cited by
-
Membrane Interactions and Toxicity by Misfolded Protein Oligomers.Front Cell Dev Biol. 2021 Mar 11;9:642623. doi: 10.3389/fcell.2021.642623. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33791300 Free PMC article. Review.
-
Amyloid Prefibrillar Oligomers: The Surprising Commonalities in Their Structure and Activity.Int J Mol Sci. 2021 Jun 16;22(12):6435. doi: 10.3390/ijms22126435. Int J Mol Sci. 2021. PMID: 34208561 Free PMC article. Review.
-
Amyloid pores in mitochondrial membranes.Neural Regen Res. 2021 Nov;16(11):2225-2226. doi: 10.4103/1673-5374.310682. Neural Regen Res. 2021. PMID: 33818503 Free PMC article. No abstract available.
-
Therapeutic Drugs and Devices for Tackling Ocular Hypertension and Glaucoma, and Need for Neuroprotection and Cytoprotective Therapies.Front Pharmacol. 2021 Sep 17;12:729249. doi: 10.3389/fphar.2021.729249. eCollection 2021. Front Pharmacol. 2021. PMID: 34603044 Free PMC article. Review.
-
Extract from the Marine Seaweed Padina pavonica Protects Mitochondrial Biomembranes from Damage by Amyloidogenic Peptides.Molecules. 2021 Mar 7;26(5):1444. doi: 10.3390/molecules26051444. Molecules. 2021. PMID: 33799979 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

