Interleukin-11 is important for vascular smooth muscle phenotypic switching and aortic inflammation, fibrosis and remodeling in mouse models
- PMID: 33082445
- PMCID: PMC7576123
- DOI: 10.1038/s41598-020-74944-7
Interleukin-11 is important for vascular smooth muscle phenotypic switching and aortic inflammation, fibrosis and remodeling in mouse models
Abstract
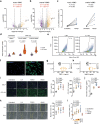
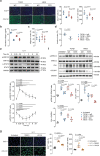
Transforming growth factor beta-1 (TGFβ1) is a major driver of vascular smooth muscle cell (VSMC) phenotypic switching, an important pathobiology in arterial disease. We performed RNA-sequencing of TGFβ1-stimulated human aortic or arterial VSMCs which revealed large and consistent upregulation of Interleukin 11 (IL11). IL11 has an unknown function in VSMCs, which highly express the IL11 receptor alpha, suggestive of an autocrine loop. In vitro, IL11 activated ERK signaling, but inhibited STAT3 activity, and caused VSMC phenotypic switching to a similar extent as TGFβ1 or angiotensin II (ANGII) stimulation. Genetic or therapeutic inhibition of IL11 signaling reduced TGFβ1- or ANGII-induced VSMC phenotypic switching, placing IL11 activity downstream of these factors. Aortas of mice with Myh11-driven IL11 expression were remodeled and had reduced contractile but increased matrix and inflammatory genes expression. In two models of arterial pressure loading, IL11 was upregulated in the aorta and neutralizing IL11 antibodies reduced remodeling along with matrix and pro-inflammatory gene expression. These data show that IL11 plays an important role in VSMC phenotype switching, vascular inflammation and aortic pathobiology.
Conflict of interest statement
S.A.C. and S.S. are co-inventors of the patent applications ‘Treatment of fibrosis’ (WO/2017/103108) and ‘IL-11 antibodies’ (WO/2018/109174). S.A.C., S.S., W.W.L. and B.N are co-inventors of the patent application ‘Treatment of SMC mediated disease’ (WO/2019/073057). S.A.C. and S.S. are co-founders and shareholders of Enleofen Bio PTE LTD, a company (which S.A.C. is a director of) that develops anti-IL11 therapeutics. All other authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

