Formyl peptide receptors in the mucosal immune system
- PMID: 33082511
- PMCID: PMC7572937
- DOI: 10.1038/s12276-020-00518-2
Formyl peptide receptors in the mucosal immune system
Abstract
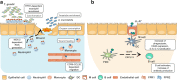
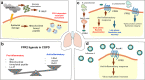
Formyl peptide receptors (FPRs) belong to the G protein-coupled receptor (GPCR) family and are well known as chemotactic receptors and pattern recognition receptors (PRRs) that recognize bacterial and mitochondria-derived formylated peptides. FPRs are also known to detect a wide range of ligands, including host-derived peptides and lipids. FPRs are highly expressed not only in phagocytes such as neutrophils, monocytes, and macrophages but also in nonhematopoietic cells such as epithelial cells and endothelial cells. Mucosal surfaces, including the gastrointestinal tract, the respiratory tract, the oral cavity, the eye, and the reproductive tract, separate the external environment from the host system. In mucosal surfaces, the interaction between the microbiota and host cells needs to be strictly regulated to maintain homeostasis. By sharing the same FPRs, immune cells and epithelial cells may coordinate pathophysiological responses to various stimuli, including microbial molecules derived from the normal flora. Accumulating evidence shows that FPRs play important roles in maintaining mucosal homeostasis. In this review, we summarize the roles of FPRs at mucosal surfaces.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. - PubMed
-
- Pommerville, J. C. Alcamo’s Fundamentals of Microbiology: Body Systems (Jones & Bartlett Publishers, 2009).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

