X Chromosome inactivation: a modifier of factor VIII and IX plasma levels and bleeding phenotype in Haemophilia carriers
- PMID: 33082527
- PMCID: PMC7868370
- DOI: 10.1038/s41431-020-00742-4
X Chromosome inactivation: a modifier of factor VIII and IX plasma levels and bleeding phenotype in Haemophilia carriers
Abstract
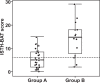
Haemophilia A and B are X-linked hemorrhagic disorders caused by gene variants in the F8 and F9 genes. Due to recessive inheritance, males are affected, while female carriers are usually asymptomatic with a wide range of factor VIII (FVIII) or IX (FIX) levels. Bleeding tendency in female carriers is extremely variable and may be associated with low clotting factor levels. This could be explained by F8 or F9 genetic variations, numerical or structural X chromosomal anomalies, or epigenetic variations such as irregular X chromosome inactivation (XCI). The aim of the study was to determine whether low FVIII or FIX coagulant activity in haemophilia carriers could be related to XCI and bleeding symptoms. HUMARA assay was performed on 73 symptomatic carriers with low clotting activity ≤50 IU/dL. Bleeding Assessment Tool (BAT) from the International Society on Thrombosis and Haemostasis (ISTH) was used to describe symptoms in the cohort of carriers. In 97% of haemophilia carriers, a specific gene variant in heterozygous state was found, which alone could not justify their low FVIII or FIX levels (≤50 IU/dL). A statistical association between XCI pattern and FVIII and FIX levels was observed. Moreover, female carriers with low coagulant activity (≤20 IU/dL) and high degree of XCI ( ≥ 80:20) had a higher ISTH-BAT score than the carriers with the opposite conditions (>20 IU/dL and <80:20). In our cohort of haemophilia carriers, XCI was significantly skewed, which may contribute to the low expression of clotting factor levels and bleeding symptoms.
Conflict of interest statement
ES is a member of advisory committees (Sobi, NovoNordisk, Roche, Shire, Bayer, Kedrion, Grifols, Pfizer) and speaker bureaus (Octapharma, Kedrion, Grifols, Bayer, Roche, Shire, Bioverativ, Sobi, NovoNordisk, Pfizer, CSL Behring). FP has received honoraria for participating as a speaker at satellite symposia organised by Bioverativ, Grifols, Roche, Sanofi, Sobi, Spark and Takeda. FP reports participation on the advisory boards of Sanofi and Sobi.
Figures

References
-
- Mannucci PM, Tuddenham EG. The Hemophilias–from royal genes to gene therapy. N Engl J Med. 2001;344:1773–9. - PubMed
-
- Peyvandi F, Garagiola I, Young G. The past and future of hemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388:187–97.. - PubMed
-
- Plug I, Mauser-Bunschoten EP, Bröcker-Vriends AH, van Amstel HK, van der Bom JG, van Diemen-Homan JE, et al. Bleeding in carriers of hemophilia. Blood. 2006;108:52–6. - PubMed
-
- Radic CP, Rossetti LC, Abelleyro MM, Tetzlaff T, Candela M, Neme D, et al. Phenotype-genotype correlations in hemophilia A carriers are consistent with the binary role of the phase between F8 and X-chromosome inactivation. J Thromb Haemost. 2015;13:530–9. - PubMed
-
- Miyawaki Y, Suzuki A, Fujimori Y, Takagi A, Murate T, Suzuki N, et al. Severe hemophilia A in a Japanese female caused by an F8-intron 22 inversion associated with skewed X chromosome inactivation. Int J Hematol. 2010;92:405–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

