Major Durable Response of Pembrolizumab in Chemotherapy Refractory Small Cell Bladder Cancer: A Case Report
- PMID: 33082749
- PMCID: PMC7548951
- DOI: 10.1159/000509747
Major Durable Response of Pembrolizumab in Chemotherapy Refractory Small Cell Bladder Cancer: A Case Report
Abstract
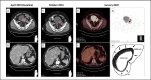
Small cell carcinoma of the urinary bladder is a rare subtype (incidence of 1-9/1,000,000), characterized by an aggressive behavior with early metastasis and poor prognosis. Chemotherapy, radiation, and surgery are the usual treatment options, but to date, no accepted standard treatment exists. Since small cell bladder cancer shares similar clinicopathological features with small cell lung cancer, the same type of chemotherapy has been used. Recently, immune checkpoint inhibitors have shown effect in small cell lung cancer, but data regarding small cell bladder cancer is insufficient. Here we present a case where a 73-year-old male with chemorefractory metastatic small cell bladder cancer received a successful treatment with immune checkpoint inhibitor pembrolizumab resulting in a major durable response and no side effects. To our knowledge, this is the second case report on successful treatment of the rare subtype of small cell bladder cancer with an immune checkpoint inhibitor, supporting the use of pembrolizumab as a therapeutic option for small cell bladder cancer. Serum neuron-specific enolase was a useful biomarker both for chemo- and immunotherapy response.
Keywords: Biomarkers; Immunotherapy; Liver metastasis; Small cell bladder carcinoma; Small cell carcinoma.
Copyright © 2020 by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- Wilde L, Ali SM, Solomides CC, Ross JS, Trabulsi E, Hoffman-Censits J, et al. Response to Pembrolizumab in a Patient With Chemotherapy Refractory Bladder Cancer With Small Cell Variant Histology: A Case Report and Review of the Literature. Clin Genitourin Cancer. 2017;15((3)):e521–e4. - PubMed
-
- Naito A, Taguchi S, Nakagawa T, Matsumoto A, Nagase Y, Tabata M, et al. Prognostic significance of serum neuron-specific enolase in small cell carcinoma of the urinary bladder. World J Urol. 2017;35((1)):97–103. - PubMed
-
- Administration . FDA approves atezolizumab for extensive-stage small cell lung cancer. U.S. 2019. [cited 2019 March 19] Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atez....
Publication types
LinkOut - more resources
Full Text Sources

