The Outcome of Hydroxychloroquine in Patients Treated for COVID-19: Systematic Review and Meta-Analysis
- PMID: 33082891
- PMCID: PMC7556078
- DOI: 10.1155/2020/4312519
The Outcome of Hydroxychloroquine in Patients Treated for COVID-19: Systematic Review and Meta-Analysis
Abstract
Background: The pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) resulted in an unprecedented public health challenge worldwide. Despite urgent and extensive global efforts, the existing evidence is inconclusive regarding the medications used for the treatment of COVID-19.
Purpose: To generate an up-to-date evidence for the clinical safety and efficacy of hydroxychloroquine (HCQ) with or without azithromycin (AZ) among patients treated for COVID-19. Data Source. PubMed, Cochrane CENTRAL, LITCOVID, Web of Science, SCOPUS, BioRxiv, Embase, MedRxiv, and Wiley online library were searched from 2019/12/30 to 2020/05/23. Study Selection. Three investigators assessed the quality of the studies. Data Extraction. Data about study characteristics, effect estimates, and the quality of the studies were extracted by two independent reviewers and cross-checked by the third reviewer. Data Synthesis. The data of 6,782 (HCQ group, 3623; HCQ + AZ group, 1,020; control group, 2139) participants were included. HCQ was compared with standard care for virologic efficacy, disease progression, mortality, and adverse effects. HCQ was also compared with HCQ + AZ for QTc prolongation, admission to the intensive care unit, and mortality. The study found HCQ did not alter the rate of virologic cure (OR = 0.78; 95% CI: 0.39-1.56) and the risk of mortality (OR = 1.26; 95% CI: 0.66-2.39). The pooled prevalence for mortality was 5.8% (95% CI: 0.9%-10.8%). Moreover, HCQ did not impact disease progression (OR = 0.9; 95% CI: 0.36-2.29) but resulted in a higher risk of adverse effects (OR = 2.35; 95% CI: 1.15-4.8). HCQ was also compared against HCQ + AZ, and no difference was observed in QTc prolongation above 500 ms (OR = 1.11; 95% CI: 0.54-2.28), admission to the intensive care unit (OR = 0.92; 95% CI: 0.52-1.63), and mortality (OR = 0.88; 95% CI: 0.55-1.43). However, in the analysis of single-arm studies, about 11.2% (95% CI: 7.0%-15.5%) of patients have developed an absolute increase of QTc greater than 500 ms, and 4.1% (95% CI: 1.1%-7.1%) of patients discontinued their medication.
Conclusion: This meta-analysis and systematic review, which included a limited number of poorly designed studies of patients with COVID-19, revealed HCQ is intolerable, unsafe, and not efficacious. Similarly, HCQ + AZ combination was not different from HCQ alone in curbing mortality and ICU admission.
Copyright © 2020 Teshale Ayele Mega et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
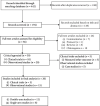











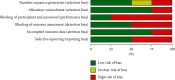
References
-
- WHO Health Emergency Coronavirus 19 Dashboard, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

