The worldwide clinical trial research response to the COVID-19 pandemic - the first 100 days
- PMID: 33082937
- PMCID: PMC7539080
- DOI: 10.12688/f1000research.26707.2
The worldwide clinical trial research response to the COVID-19 pandemic - the first 100 days
Abstract
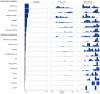
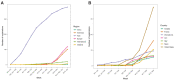
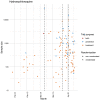
Background: Never before have clinical trials drawn as much public attention as those testing interventions for COVID-19. We aimed to describe the worldwide COVID-19 clinical research response and its evolution over the first 100 days of the pandemic. Methods: Descriptive analysis of planned, ongoing or completed trials by April 9, 2020 testing any intervention to treat or prevent COVID-19, systematically identified in trial registries, preprint servers, and literature databases. A survey was conducted of all trials to assess their recruitment status up to July 6, 2020. Results: Most of the 689 trials (overall target sample size 396,366) were small (median sample size 120; interquartile range [IQR] 60-300) but randomized (75.8%; n=522) and were often conducted in China (51.1%; n=352) or the USA (11%; n=76). 525 trials (76.2%) planned to include 155,571 hospitalized patients, and 25 (3.6%) planned to include 96,821 health-care workers. Treatments were evaluated in 607 trials (88.1%), frequently antivirals (n=144) or antimalarials (n=112); 78 trials (11.3%) focused on prevention, including 14 vaccine trials. No trial investigated social distancing. Interventions tested in 11 trials with >5,000 participants were also tested in 169 smaller trials (median sample size 273; IQR 90-700). Hydroxychloroquine alone was investigated in 110 trials. While 414 trials (60.0%) expected completion in 2020, only 35 trials (4.1%; 3,071 participants) were completed by July 6. Of 112 trials with detailed recruitment information, 55 had recruited <20% of the targeted sample; 27 between 20-50%; and 30 over 50% (median 14.8% [IQR 2.0-62.0%]). Conclusions: The size and speed of the COVID-19 clinical trials agenda is unprecedented. However, most trials were small investigating a small fraction of treatment options. The feasibility of this research agenda is questionable, and many trials may end in futility, wasting research resources. Much better coordination is needed to respond to global health threats.
Keywords: COVID-19; clinical research agenda; hydroxychloroquine.
Copyright: © 2020 Janiaud P et al.
Conflict of interest statement
No competing interests were disclosed.
Figures

References
-
- WHO: Pneumonia of unknown cause – China. WHO. Accessed May 26, 2020. Reference Source
-
- Yan W: Coronavirus Tests Science’s Need for Speed Limits. The New York Times. Accessed May 26, 2020. Reference Source
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

