Depicting the battle between nectarine and Monilinia laxa: the fruit developmental stage dictates the effectiveness of the host defenses and the pathogen's infection strategies
- PMID: 33082973
- PMCID: PMC7527454
- DOI: 10.1038/s41438-020-00387-w
Depicting the battle between nectarine and Monilinia laxa: the fruit developmental stage dictates the effectiveness of the host defenses and the pathogen's infection strategies
Abstract
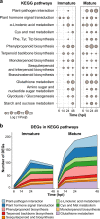
Infections by the fungus Monilinia laxa, the main cause of brown rot in Europe, result in considerable losses of stone fruit. Herein, we present a comprehensive transcriptomic approach to unravel strategies deployed by nectarine fruit and M. laxa during their interaction. We used M. laxa-inoculated immature and mature fruit, which was resistant and susceptible to brown rot, respectively, to perform a dual RNA-Seq analysis. In immature fruit, host responses, pathogen biomass, and pathogen transcriptional activity peaked at 14-24 h post inoculation (hpi), at which point M. laxa appeared to switch its transcriptional response to either quiescence or death. Mature fruit experienced an exponential increase in host and pathogen activity beginning at 6 hpi. Functional analyses in both host and pathogen highlighted differences in stage-dependent strategies. For example, in immature fruit, M. laxa unsuccessfully employed carbohydrate-active enzymes (CAZymes) for penetration, which the fruit was able to combat with tightly regulated hormone responses and an oxidative burst that challenged the pathogen's survival at later time points. In contrast, in mature fruit, M. laxa was more dependent on proteolytic effectors than CAZymes, and was able to invest in filamentous growth early during the interaction. Hormone analyses of mature fruit infected with M. laxa indicated that, while jasmonic acid activity was likely useful for defense, high ethylene activity may have promoted susceptibility through the induction of ripening processes. Lastly, we identified M. laxa genes that were highly induced in both quiescent and active infections and may serve as targets for control of brown rot.
Keywords: Biotic; Fungal genetics; Transcriptomics.
© The Author(s) 2020.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures




References
-
- Rungjindamai N, Jeffries P, Xu XM. Epidemiology and management of brown rot on stone fruit caused by Monilinia laxa. Eur. J. Plant Pathol. 2014;140:1–17. doi: 10.1007/s10658-014-0452-3. - DOI
-
- Martini, C. & Mari, M. Monilinia fructicola, Monilinia laxa (Monilinia Rot, Brown Rot). In (ed. Silvia Bautista-Baños) Postharvest Decay: Control Strategies 233–265 (Elsevier, 2014).
-
- RosBREED. RosBREED peach brown rot. https://www.rosbreed.org/node/424 (Accessed January 16, 2020).
-
- Usall J, Casals C, Sisquella M, Palou L, De Cal A. Alternative technologies to control postharvest diseases of stone fruits. Stewart Postharvest Rev. 2015;11:1–6.
-
- Byrne, D. H. Fruit Breeding. Fruit Breeding, Handbook of Plant Breeding (Springer US, 2012).

