Vaccination with the recombinant major outer membrane protein elicits long-term protection in mice against vaginal shedding and infertility following a Chlamydia muridarum genital challenge
- PMID: 33083025
- PMCID: PMC7530680
- DOI: 10.1038/s41541-020-00239-7
Vaccination with the recombinant major outer membrane protein elicits long-term protection in mice against vaginal shedding and infertility following a Chlamydia muridarum genital challenge
Abstract
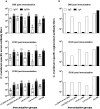
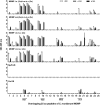
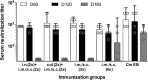
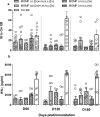
Implementation of a vaccine is likely the best approach to curtail Chlamydia trachomatis infections. The aim of this study was to determine the ability of a vaccine formulated with the recombinant major outer membrane protein (MOMP) and Th1 and Th2 adjuvants, delivered by combinations of systemic and mucosal routes, to elicit long-term protection in mice against a genital challenge with Chlamydia muridarum. As a negative control, mice were vaccinated with the recombinant Neisseria gonorrhoeae porinB, and the positive control group was immunized with C. muridarum live elementary bodies (EB). The four vaccines formulated with MOMP, as determined by the titers of IgG and neutralizing antibodies in serum, proliferative responses of T-cells stimulated with EB and levels of IFN-γ in the supernatants, elicited robust humoral and cellular immune responses over a 6-month period. Groups of mice were challenged genitally at 60, 120, or 180 days postimmunization. Based on the number of mice with positive vaginal cultures, number of positive cultures, length of time of shedding, and number of inclusion forming units recovered, MOMP vaccinated groups were significantly protected. To assess fertility, when the vaginal cultures became negative, female mice were caged with male mice and the outcome of the pregnancy evaluated. As determined by the number of pregnant mice and the number of embryos, two of the vaccine formulations protected mice up to 180 days postimmunization. To our knowledge this is the first subunit of Chlamydia vaccine that has elicited in mice significant long-term protection against a genital challenge.
Keywords: Diseases; Immunology; Microbiology.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures


Similar articles
-
Increased immunoaccessibility of MOMP epitopes in a vaccine formulated with amphipols may account for the very robust protection elicited against a vaginal challenge with Chlamydia muridarum.J Immunol. 2014 Jun 1;192(11):5201-13. doi: 10.4049/jimmunol.1303392. Epub 2014 Apr 28. J Immunol. 2014. PMID: 24778450 Free PMC article.
-
A vaccine formulated with the major outer membrane protein can protect C3H/HeN, a highly susceptible strain of mice, from a Chlamydia muridarum genital challenge.Immunology. 2015 Nov;146(3):432-43. doi: 10.1111/imm.12520. Epub 2015 Oct 1. Immunology. 2015. PMID: 26423798 Free PMC article.
-
The cationic liposomal adjuvants CAF01 and CAF09 formulated with the major outer membrane protein elicit robust protection in mice against a Chlamydia muridarum respiratory challenge.Vaccine. 2017 Mar 23;35(13):1705-1711. doi: 10.1016/j.vaccine.2017.02.020. Epub 2017 Feb 24. Vaccine. 2017. PMID: 28238632
-
A vaccine formulated with a combination of TLR-2 and TLR-9 adjuvants and the recombinant major outer membrane protein elicits a robust immune response and significant protection against a Chlamydia muridarum challenge.Microbes Infect. 2014 Mar;16(3):244-52. doi: 10.1016/j.micinf.2013.11.009. Epub 2013 Nov 27. Microbes Infect. 2014. PMID: 24291713 Free PMC article.
-
Induction of protection against vaginal shedding and infertility by a recombinant Chlamydia vaccine.Vaccine. 2011 Jul 18;29(32):5276-83. doi: 10.1016/j.vaccine.2011.05.013. Epub 2011 May 24. Vaccine. 2011. PMID: 21609745 Free PMC article.
Cited by
-
Evaluation of Four Adjuvant Combinations, IVAX-1, IVAX-2, CpG-1826+Montanide ISA 720 VG and CpG-1018+Montanide ISA 720 VG, for Safety and for Their Ability to Elicit Protective Immune Responses in Mice against a Respiratory Challenge with Chlamydia muridarum.Pathogens. 2023 Jun 22;12(7):863. doi: 10.3390/pathogens12070863. Pathogens. 2023. PMID: 37513710 Free PMC article.
-
Immune signature of Chlamydia vaccine CTH522/CAF®01 translates from mouse-to-human and induces durable protection in mice.Nat Commun. 2024 Feb 23;15(1):1665. doi: 10.1038/s41467-024-45526-2. Nat Commun. 2024. PMID: 38396019 Free PMC article.
-
Th1/Th17 T cell Tissue-Resident Immunity Increases Protection, But Is Not Required in a Vaccine Strategy Against Genital Infection With Chlamydia trachomatis.Front Immunol. 2021 Dec 2;12:790463. doi: 10.3389/fimmu.2021.790463. eCollection 2021. Front Immunol. 2021. PMID: 34925371 Free PMC article.
-
Induction of Protection in Mice against a Chlamydia muridarum Respiratory Challenge by a Vaccine Formulated with the Major Outer Membrane Protein in Nanolipoprotein Particles.Vaccines (Basel). 2021 Jul 7;9(7):755. doi: 10.3390/vaccines9070755. Vaccines (Basel). 2021. PMID: 34358171 Free PMC article.
-
Determination of the safety and efficacy of recombinant Chlamydia muridarum MOMP vaccines, formulated with CpG-1826 and 70%, 50%, 30% or 10% concentrations of Montanide ISA-720 VG, to elicit protective immune responses against a C. muridarum respiratory challenge.Res Sq [Preprint]. 2023 Dec 11:rs.3.rs-3688658. doi: 10.21203/rs.3.rs-3688658/v1. Res Sq. 2023. Update in: NPJ Vaccines. 2024 Jun 10;9(1):104. doi: 10.1038/s41541-024-00880-6. PMID: 38168233 Free PMC article. Updated. Preprint.
References
-
- CDC. Division of STD Prevention 1–168 (U.S. Department of Health and Human Services, Atlanta, 2019).
-
- Schachter, J. & Dawson, C. R. Human Chlamydial Infections. (PSG Publishing Company, Burlington, 1978).
-
- Stamm, W. in Sexually Transmitted Diseases (eds. Sparling, P. F. et al.) 575–593 (McGrawHill Book Co., Pennsylvania, 2008).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

